This study assesses the effects of vegetation patterns and environmental factors on the abundance of natural tree and shrub regeneration in semi-arid forests of the Zagros Mountains, western Iran. We sampled 120 releves at different topographic positions in a protected area of the studied region. Floristic composition, slope, elevation and soil properties were recorded at each releve, and woody seedling density was measured. We have first discerned five floristic groups using two-way indicator species analysis (TWINSPAN), detrended correspondence analysis (DCA), and canonical correspondence analysis (CCA) and then explored the relationships among the floristic group compositions, environmental factors and seedling densities. The indicator species of the five groups were Quercus brantii, Acer monspessulanum, Cerasus microcarpa, Rhamnus arvensis and Astragalus licyoides. Our results indicated that these groups were significantly affected by elevation and soil properties and the soil properties refer to: EC (electrical conductivity), N (nitrogen), K (potassium), OM (organic matter), and bulk density. Woody regeneration was composed of Q. brantii, A. monspessulanum, C. microcarpa, Amygdalus scoparia and Crataegus pontica seedlings. The highest density of seedlings was found for Q. brantii (97.14 (±48.00) plants/hm2) and the lowest for A. scoparia (2.28 (±1.50) plants/hm2). Quercus brantii was the dominant species and the seedling density was positively correlated with soil pH and P (phosphorus) values. Amygdalus scoparia regeneration was negatively correlated with elevation, and the seedling density peaked in C. microcarpa group. There was no significant variation in distribution of C. pontica seedlings among the groups, but the seedling density of this species was positively correlated with slope and K . Cerasus microcarpa seedlings were more abundant in the Q. brantii group than in other groups. This study showed that the regeneration of tree and shrub species was unequally distributed in different floristic groups for some species ( A. scoparia and C. microcarpa) but not for other ( Q. brantii and C. pontica) and was generally correlated with some environmental factors, particularly elevation, slope and soil nutrients (P and K). These results are a first step to implement future management and restoration strategies for promoting forest regeneration.
The Zagros Mountains in western Iran form its largest mountain range in Iran. This area contains various ecosystems, prominent among which are forests in a semi-arid climate. These seasonal dry forests provide a home and livelihood for about 10% of the country’ s population (Salehi et al., 2010). But, they undergo increasingly strong anthropogenic pressure, causing severe degradation of these ecosystems. More than 1.7× 106 hm2 of the forests in the Zagros Mountains (namely Zagros forests) have been deforested since 1962. Studies suggest that increasing population, low level of development, and high dependency of local communities on the forests for their primary livelihoods are the main reasons for the deforestation (Ghazanfari et al., 2004; Heydari et al., 2016).
The natural regeneration of these forests is very slow, thus jeopardizing the ecological services of these ecosystems. Different reasons are offered to explain this poor regeneration, and many studies have emphasized the role of overgrazing pressure limiting woody species establishment in the Zagros Mountains (Jazirehi and Ebrahimi, 2003; Pourhashemi et al., 2004; Sagheb-Talebi et al., 2004). Unexpectedly, the lack of natural regeneration is also observed even in the Zagros forests that enjoy protected status, where grazing pressure and other anthropogenic disturbances are minimal (Pourhashemi et al., 2004).
This observation points to the involvement of other factors. The influence of the main physiographic variables on the regeneration process has been largely documented, and numerous studies have emphasized the role of elevation, slope and topography as key factors influencing above- and below-ground conditions, and thereby regeneration success (e.g., Peterson and Pickett, 1990; van Mantgem et al., 2006; Martí n-Alcó n and Coll, 2016). The relationships linking regeneration and soil physical and chemical properties have also been explored (e.g., Kabrick et al., 2005; Hattori et al., 2013), although our understanding of them is still limited (Dickie et al., 2007). In Mediterranean regions, previous studies have shown that soil properties such as texture (Ibá ñ ez et al., 2014), phosphorus availability (Sardans et al., 2005; Pascual et al., 2012), potassium availability (Gó mez-Aparicio et al., 2005; Sardans et al., 2005) and nitrogen availability (Sardans et al., 2006) often strongly influence tree regeneration. However, the natural regeneration of woody species in different ecological species groups has been under-researched.
Ecological species groups refer to the plants that share similar affinities to environmental conditions and tend to occupy the same ecological niches across the landscape (Adel et al., 2014). By integrating soil and physiographic information, ecological species grouping offers an effective way to devise a land classification that can then be used to support vegetation mapping and management strategies (Host and Pregitzer, 1991). There is accordingly a need to know more about the relationships between ecological plant species groups and patterns of regeneration (composition, density) of the main woody species in semi-arid regions.
This study was designed to fill this gap. We hypothesized that the natural regeneration of shrub and tree species varies with floristic compositions of the communities and environmental factors (including soil physical and chemical properties). To test this hypothesis, we adopted the following approach: (i) we first produced a floristic classification of the main communities occurring in our study area, and explored the correlations between those communities and environmental factors, and (ii) we then studied woody seedling density and composition in the different communities and examined the links between regeneration and environmental factors.
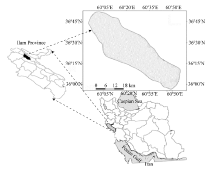
The study area was a natural forest on Manesht-Ghalarang, a protected area of the Zagros forests in Ilam Province of western Iran (36º 00′ -36º 45′ N, 60º 05′ -60º 50′ E; Fig. 1). The elevation ranges from 1400 to 2200 m a.s.l. and slope from 5º to 75º . Slope aspects were mainly north- and south-facing. The dominant species was the Persian oak (Quercus brantii), which made up nearly 90% of the overstorey and was accompanied with a few other tree species such as Pistacia atlantica, Crataegus pontica, and Acer monspessulanum. The understorey comprised diverse plant species. In total, 52 families, 156 genera and 231 plant species have been found in this area. The largest family is Asteraceae with 18 genera and 29 species, and the largest genus is Astragalus with 11 species (Darvishnia et al., 2012). Soils were calcic/calcareous, the overall texture was sandy clay, and the dominant parent rock was limestone. The climate was semi-arid: mean annual rainfall, maximum temperature and minimum temperature were 320 mm, 28° C and 5.8° C, respectively (Mirzaei, 2016).
In the study area, we set up 19 transects with lengths ranging from 700 to 1400 m from lower to upper hillside. Totally, we sampled 120 plots (20 m× 20 m each). The location of transects and releves were selected so as to represent a wide range of physiographic and environmental variations. In each sampling plot, elevation, aspect and slope were recorded in the field using GPS. A floristic releve was then taken on each plot: each vascular species was identified and named according to the available literature (Mozaffarian, 2008). Abundance-dominance for each species was estimated on the Braun-Blanquet scale (Mü ller-Dombois and Ellenberg, 1974).
To study the physical and chemical properties of soils, three composite soil samples were collected at depth 0-20 cm near the centre of each plot (120 samples). These soil samples were placed in plastic bags and sent immediately to the laboratory for soil analysis.
Soil samples were air-dried and sieved to 2 mm meshes, and analyzed for soil texture, electrical conductivity (EC), pH, bulk density, nitrogen (N), phosphorus (P), potassium (K), carbon (C) and organic matter (OM). The soil texture was measured hydrometrically, the bulk densities gravimetrically. The soil EC and pH were measured in deionized water (McLean, 1982). Total N was determined by the Kjeldahl method (Bremner, 1996). Available P was determined according to the method of Watanabe and Olsen (Watanabe and Olsen, 1965). Exchangeable K was analyzed using inductively coupled plasma atomic emission spectroscopy (Kalra and Maynard, 1991). Total OM was determined using a wet oxidation technique (Walkley and Black, 1934). The abundance and composition of natural tree and shrub regeneration were recorded by establishing a 10 m× 10 m central subplot on each sampling plot. On each subplot, the number of seedlings and samplings of tree and shrub species that were ≥ 5 cm tall and < 1 cm diameter at breast height were recorded (hereafter referred to as ‘ seedlings’ ).
To draw up a floristic typology of our area as a framework for further analysis, a classification was undertaken using two-way indicator species analysis (TWINSPAN, Hill, 1979) on a quantitative data matrix comprising 120 releves and 211 species after discarding species with three or fewer occurrences. Each plant of the releve was given a coefficient of abundance-dominance (from 1 to 5) that reflects the cover of the plant, i.e., 1: < 5%, 2: 5%-25%, 3: 25%-50%, 4: 50%-75%, 5: > 75%. Classification was truncated at the fourth division after examination of the resulting groups and complementary ordination by the detrended correspondence analysis (DCA; Hill and Gauch Jr, 1980). This analysis resulted in five vegetation groups (i.e., plant communities), which were named after their dominant species. In order to detect correlations of these vegetation groups with environmental factors (slope, elevation and soil properties), canonical correspondence analysis (CCA) was undertaken using a second matrix formed by 120 releves and the 13 environmental factors considered. Relationships between the ordination axes and community and environmental variables were tested using Pearson’ s simple linear correlation coefficient. The means and standard errors of the seedling densities (plants/hm2), and environmental variables were calculated for the five groups, and statistical differences were tested using ANOVA and Duncan’ s multiple comparison tests.
Pearson’ s correlation was used to determine the correlation coefficients between soil and physiographic factors and seedling densities. Statistical analyses were carried out in SPSS (version 16.0) and PC-ORD program (version 4.17).
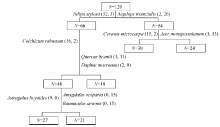
From the results of vegetation classification using TWINSPAN, the 120 plots were classified into five groups (Fig. 2). The indicator species of the clustering dendrogram for the successive divisions are as follows: first division: Tulipa stylosa on the left and Aegilops triuncialis on the right; second division: Colchicum robustum (left), and Quercus brantii and Daphne macronata (right); third division: Cerasus microcarpa (left) and Acer monspessulanum (right); fourth division: Astragalus licyoides (left) and Amygdalus scopariaandRanunculus arvensis (right).
According to the eigenvalue of each division, the vegetation in the study area was classified into five ecological species groups named after their dominant species: Group 1: C. microcapa; Group 2: Q. brantii; Group 3: A. monspessulanum; Group 4: A. scoparia; Group 5: A. licyoides.
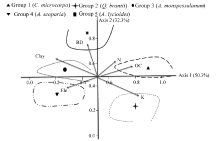
The detrended correspondence analysis (DCA) was used to search for major gradients in species compositions and to describe the general pattern of species distribution along the environmental gradients. It was found that the first and second axes explained respectively 63.0% and 35.3% of the total variation (Fig. 3). The first axis separated the first, third and fourth groups, while the second axis separated the second and fifth groups. The application of DCA thus confirmed the separation between these communities that was indicated by the TWINSPAN analysis.
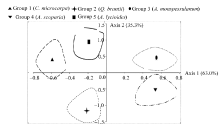 | Fig. 3 Detrended correspondence analysis ordination diagram based on plant species. The numbers in parentheses indicate the percentage of total variance explained by each axis. |
Canonical correspondence analysis (CCA) was used to analyze the relationship between species composition and the environmental variables (Fig. 4). The percentage variances of the species-environment relation for axes of CCA (and their eigenvalues) were 50.3% and 32.3% for axes 1 and 2, respectively. The species-environment correlation coefficients calculated for the first two axes of CCA were 0.91 and 0.79. The highest coefficients with the first CCA axis were found for soil organic carbon (0.68; P< 0.01), K (0.41; P< 0.05), elevation (-0.78; P< 0.01), N (0.51; P< 0.05) and clay (-0.46; P< 0.05). The second CCA axis was closely correlated with bulk density (0.89; P< 0.01).
The CCA results showed that soil nitrogen and organic carbon were the most important factors in the first group. Potassium was the separation (deciding) factor in the second group. The third and fourth groups were formed around the left side of the first axis. Clay and elevation were higher in the third and fourth groups. Soil bulk density was the separation (deciding) factor in the fifth group (Fig. 4).
Table 1 shows significant differences between the different groups in elevation (P< 0.001) and some soil parameters: bulk density (P=0.002), EC (P< 0.001), N (P=0.021), OM (P< 0.001) and K (P< 0.001). Group 4 (A. scoparia) was at the highest and Group 2 (Q. brantii) at the lowest elevation above sea level. Soil bulk density was highest for Group 5 and lowest for Group 1 (Table 1). Group 1 showed the highest OM, C and N values. The K content was higher in Group 2 (Q. brantii) than in the other groups (Table 2).
| Table 1 Elevation, slope, clay, sand, silt, bulk density and pH values for the different groups |
| Table 2 Soil EC and chemical properties for the different groups |
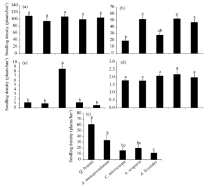
Five tree and shrub species regenerated naturally in the Manesht-Ghalarang region includingQ. brantii, A. monspessulanum, A. scoparia, C. ponticaandC. microcarpa. The results show that Q. brantii(mean=97.14 plants/hm2) and A. scoparia (mean=2.28 plants/hm2) had respectively the highest and lowest seedling densities (Table 3).
| Table 3 Mean and standard deviation of seedling density values for the different groups |
Density of Q. brantii seedlings did not vary significantly among the ecological species groups (Fig. 5a). By contrast, regeneration of A. monspessulanum, the second most abundant regenerating species in the study site (mean=40.91 plants/hm2) peaked in A. monspessulanumspecies group (Fig. 5b). A. scoparia, the least abundant regenerating species (mean=2.28 plants /hm2), was most abundant in the C. microcarpa group (Fig. 5c), whereas no preferential regeneration of C. pontica was found among the five groups (Fig. 5d). Last, the regeneration of C. microcarpa was highest in the Q. brantii group (Fig. 5e).
Pearson’ s correlation analysis showed significant correlations between natural regeneration and ome environmental factors (Table 4). Specifically, Q. brantiiregeneration was found preferentially on soils with higher pH and higher P content. Acer monspessulanumseedlings were more numerous at higher elevations on steeper slopes, and on sandy soils with higher K content. By contrast, A. scoparia regeneration was less abundant at high elevation, whereas regeneration of C. microcarpaseedlings was positively correlated with soil sand content and negatively with soil bulk density. Last, seedling density of C. ponticaincreased with the increases in slope and soil K content (Table 4).
| Table 4 Matrix showing Pearson’ s correlation (r) between topography and soil physical-chemical properties and natural regeneration of tree and shrub species |
The results of this study show that natural regeneration in the study area was quite low even though this area was protected. Anthropogenic disturbances, such as livestock grazing, overexploitation of the firewood resource, agriculture in the forest understorey or fires, were largely minimized in the study area. We found that Q. brantii seedlings were the most abundant among the different shrub and tree species in the Persian oak forests. Quercus brantii seedlings distribution can be explained by soil P content. This finding is consistent with previous studies reporting a significant relationship between P content and establishment of different Mediterranean oak species (Gó mez-Aparicio et al., 2005, 2008). Mirzaei et al. (2007) and Basiri (2011) also noted a more abundant Q. brantii or Q. libani regeneration with increasing K soil content. These previous results are in line with previous observations indicating that P and K (rather than N or C) are more limiting factors in Mediterranean forests and shrublands (Gó mez-Aparicio et al., 2005; Sardans et al., 2005). These elements (P and K) were shown to have enhanced the water-use efficiency under drought conditions, thus improving the seedling survival and growth (Bradbury and Malcolm, 1977; Sardans et al., 2005). Seedling density of A. monspessulanum was greater at high elevations on steep slopes, i.e., the conditions where human disturbances are minimal and climatic conditions are milder. It means that the high-elevation and steep-slope conditions provide A. monspessulanumseedlings with shelter against herbivory and human disturbances. This result is consistent with other studies on tree species in dry regions (Mahoney and Rood, 1998; Taylor et al., 1999). Park (2001) also observed that regeneration of Pinus durangensis, P. teocote and Q. crassifolia was more abundant on steep-sloped sites with stony soils, while Mirzaei et al. (2007) noted a more abundant recruitment of woody species regeneration in habitats of the Zagros region with northern exposure. Explaining the positive influence of soil sand content on regeneration of this species (and also C. microcarpa) is not straightforward. Water-holding capacity is lower in a sandy texture than in fine textures (e.g., clayey or silty), but a higher sand content can also imply a more favourable soil porosity, allowing better exploration of the root system or easier water percolation to deeper soil layers, thus ensuring the availability of water in the dry season (Ibá ñ ez et al., 2014).
The natural regeneration of A. scoparia in our study area was very low (mean=2.28 plants/hm2). A similar finding was also reported by Marvie-Mohadjer (2005) and Mirzaei et al. (2007) in the same region. According to these authors, the lack of recruitment is because this species produces seeds that do not easily absorb water owing to an impermeable seed case, and water absorption (and so seed germination) is particularly limited in soils with high bulk density and low litter content. We found that the abundance of A. scopariaseedlings decreased with elevation. This can be explained by the preferential distribution of the mother trees at low elevations, whereas the dissemination of this heavy-seeded species is limited at higher elevations (Marvie-Mohadjer, 2005).
In this study we found a robust positive correlation between soil sand content and C. microcarpaseedling density, but a negative correlation with soil bulk density. Increasing soil bulk density causes a decrease in soil porosity and soil permeability, thus reducing available soil water content. These conditions are unfavourable to species recruitment (Rab, 1996) as observed for instance for P. tabulaeformis(Zhao et al., 2006).
The seedlings of C. pontica were preferentially found on steep slopes. This species produces relatively thin seeds that can be readily dispersed by wind and is thus able to colonize habitats located on steep slopes. Similarly, Tsitsoni (1997) found that slope is also an important factor explaining the natural regeneration of forests of the Kassandra Peninsula (northern Greece).
Finally, we want to point out that human-disturbance legacies in arid or semi-arid landscapes may remain over a long period (decades or even centuries) owing to the naturally low soil fertility (e.g., Swetnam et al., 1999; Ko et al., 2011). These legacies may also be somewhat related to the observed slow regeneration processes even after an arid or semi-arid landscape has been under the protection. However, no attention was paid to human-disturbance legacy issues and their possible association with slow regeneration processes.
The results of vegetation classification using TWINSPAN identified five ecological species groups and the grouping was mainly dependent on topographic factor (e.g., elevation and slope) and soil properties (chemical and physical). In this study, ecological groups were used to assess the regeneration of some representative valuable tree and shrub species. Despite the protected status of the area, we found a slow rate of regeneration for all the species. In dry landscapes, recruitment of woody species is often episodic and related to a conjunction of favourable events that occur only for short time periods. When examining the abundance of the regeneration in the different ecological species groups we found a species-specific response. That is, seedling density was equally distributed among the different groups for some species (Q. brantii, C. pontica), but not for others (A. scoparia, C. microcarpa). We showed that the abundance of regeneration was controlled by specific environmental factors and topographic factors (e.g., elevation and slope), soil properties (e.g., bulk density, sand content, K and P conents) were the major ones controlling the regeneration success. These results can help to assess the suitability of sites for the development of woody species and can thus assist in developing forest restoration strategies.
We thank Dr. Morteza POURREZA and Dr. Yahya KOOCH for their critical reading of our manuscript, and Dr. Masoumeh NOUROZI for technical support.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|