Ferula spp. are traditional medicinal plants found in arid land. Large-scale excavation for extracting bioactive compounds from the plants in arid regions of Xinjiang over the last few years has, however, significantly decreased their distributions. Due to the urgent need for preservation of these plant resources, along with the need of searching for alternative source of the useful metabolites, it is important to screen the endophytic microbial resources associated with the plant Ferula sinkiangensis K. M. Shen. In the study, a total of 125 endophytic bacteria belonging to 3 phyla, 13 orders, 23 families, and 29 genera were isolated based on 16S rRNA gene sequencing data. Among the different isolates, three strains isolated from roots were potential novel species of the genera Porphyrobacter, Paracoccus and Amycolatopsis. In this study, 79.4% and 57.1% of the total isolates were capable of producing indole-3-acetic acid (IAA) and siderophore, respectively. And, 40.6% of the strains inhibit the growth of fungal pathogen Alternaria alternata, 17.2% and 20.2% strains were positive for antagonism against Verticillium dahlia 991 and V. dahlia 7, respectively. These results demonstrated that F. sinkiangensis is a rich reservoir of endophytic bacterial resources with potential for production of biologically important functions such as plant growth-promoting factors.
An endophyte, or more particularly endophytic bacteria, is an endosymbiont that lives within a plant for at least a part of its life cycle without causing apparent disease (Hallmann et al., 1998). It has been demonstrated that many endophytes are advantageous to host plants in a mechanism similar to most plant growth-promoting rhizobacteria by either providing plant growth promoting (PGP) factors or protecting against insect, pest or plant pathogens (Feng et al., 2006; Vurukonda et al., 2016; Wang et al., 2016). The major PGP factors produced by endophytic bacteria include phytohormones such as auxins and indole-3-acetic acid (IAA), enzymes involved in growth regulation and siderophore (Boukhalfa and Crumbliss, 2002). They can also enhance plant growth by fixing atmospheric nitrogen and/or solubilizing insoluble inorganic phosphates (Triplett, 1996; Wang et al., 2009). In fact, biological nitrogen fixation and phosphate solubilization processes are considered an important PGP mechanism as these processes convert the abundantly available, unutilizable atmospheric nitrogen and phosphorus into accessible forms (Meunchang et al., 2006; Nimaichand et al., 2016). The study of endophytic bacteria and its interaction with the host is an important means to understand their ecological functions and explore their potentials for biotechnological applications, e.g., preparation of eco-friendly agricultural bioinoculants (Weller, 1988; Emmert and Handelsman, 1999; Bloemberg and Lugtenberg, 2001).
Endophytic bacteria ubiquitously inhabit most of the plant species and have been isolated from a variety of plants (Lodewyck et al., 2002). However, with relation to plants in extreme environments, limited studies have been done to explore the world of endophytic bacteria (Liu et al., 2016). Xinjiang of China is a typical arid environment where twenty-six varieties of Ferula spp., halophytic plants with medicinal properties, are found (Pimenov and Leonov, 2004). Despite the rich diversity of Ferulaspp., Ferula sinkiangensis K. M. Shen is in threat of extinction due to overutilization and other man-made destruction. Since endophytes adapted to the extremely arid environment are most likely to possess special functions (Qin et al., 2009; He et al., 2015), it is important to explore its biological natures.
Current understanding on endophytic bacteria and their community structure on plant tissues is a result of culture-based microbial diversity analysis. But majority of the naturally occurring bacteria fail to be cultured, therefore limiting our knowledge on the functional aspect of the total bacterial community. On the other hand, culture-independent studies of microbial community provide less bias information on bacterial community structure and their functional roles in the ecology. Despite the limitation of culture-based approaches, it has an advantage over the culture-independent method in that pure cultures can be utilized for biotechnological applications. The present study involved the isolation of endophytes from different tissues of F. sinkiangensis and analyzed the distribution pattern among the plant tissues. In addition, the plant growth promoting traits and their bio-control potential were studied.
Six plant samples of F. sinkiangensis were collected from three sites in Shihezi, Xinjiang (44° 08ʹ N, 86° 52ʹ E; 836 m a.s.l.) on 15 August 2015. These three sites were separated by a distance of at least 500 m from each other. The samples were rinsed thoroughly in running tap water within 24 h to exclude samples with symptoms of disease or superficial damage. After proper washing, the plant samples were separated into stems and roots (Ferula sp. is perennial, monocarpic and ephemeroid plants, only stems and roots are remained during the sampling period because of dormancy).
A 3-step surface sterilization procedure (1-min wash in 75% ethanol, 8-min wash in 5% NaOCl and subsequent rinsing in sterile distilled water for five times) was adopted for each tissue (Liu et al., 2016). To check the effectiveness of the surface-sterilization, we plated the distilled water of the final rinse onto yeast extract-malt extract agar (ISP 2, pH 7.2) plate and incubated at 30º C. The sterilization was considered complete if no microbial growth was observed on the medium.
Sterilized samples were air dried for 2 days at room temperature and were aseptically homogenized by sterilized commercial blender (Joyoung, JYL-C012). The homogenized tissue was diluted with sterile water to give a final concentration of 10-2-10-4, followed by plating 40 µ L of the tissue suspension onto the media M1 to M8 and M10 as defined by Wang (2015). The isolation plates were incubated at 30° C for 2 to 8 weeks or until bacterial growth was observed. All experiments were done in triplicate. Pure cultures obtained in the isolation media were grown and maintained in ISP 2 agar.
DNA was extracted from the endophytic bacteria following the procedures described by Araú jo et al. (2002). Amplification of the 16S rRNA gene was done using the primer pair 27F-1492R (27F: 5'-CAGAGTTTGATCCTGGCT-3'; 1492R: 5'-AGGAGGTGATCCAGCCGCA-3') (Polz and Cavanaugh, 1998) procured from Sangon Biotech (Shanghai, China). The PCR mixture (50 μ L) contained 1 μ L DNA template, 0.5 U Taq DNA polymerase, 5 μ L 10× buffer, 4 μ L dNTP Mix (100 μ M each) and 0.5 μ L of each primer (0.25 μ M). Amplification of the 16S rRNA genes was performed in a thermal cycler according to the following steps: 94º C initial denaturation for 10 min, followed by 32 cycles of denaturation at 94º C for 45 s, annealing at 56º C for 30 s and extension at 72º C for 1 min 30 s, and a final extension at 72º C for 10 min. The amplified products were purified and sequenced by Sangon Biotech (Shanghai). The sequences obtained were identified using the EzTaxon database and the reference sequences of type species retrieved from the NCBI database. Multiple sequence alignments and genetic distance calculations were done using CLUSTAL_X program. A sequence similarity of less than 98.65% with the validly published type strains was considered to be a putative novel species (Kim et al., 2014). A neighbor-joining phylogenetic dendrogram based on the 16S rRNA gene sequences was then generated using the MEGA 5.1 software package (Tamura et al., 2011).
Inorganic phosphate solubilization assay was done using the method described by dos Santos Hara and de Oliveira (2004) and Wang (2015). Bacteria were grown in a modified phosphate-solubilizing medium (10 g/L glucose, 0.5 g/L (NH4)2SO4, 0.1 g/L MgSO4• 7H2O, 0.5 g/L yeast extract, 0.2 g/L KCl, 0.2 g/L NaCl, 0.002 g/L FeSO4• 7H2O, 0.002 g/L MnSO4• 7H2O, 5.0 g/L Ca3(PO4)2, 0.025 g/L bromophenol blue, 20 g/L agar, pH 7.0). Development of a halo zone around the bacterial colony after 7-10 days incubation at 28º C was considered as a positive result for phosphate solubilization. The diameters of the halo and colony were measured with a digital caliper and the phosphate solubilizing activity was described as phosphorus solubilizing index (PSI), and the equation can be delineated as PSI=d1(halo)/d2(colony) (dos Santos Hara and de Oliveira, 2004). All experiments were done in triplicate. Solubilization was classified as low (PSI< 2), moderate (2≤ PSI≤ 3), or high (PSI> 3).
For assessment of nitrogen fixation activity, bacteria were inoculated in both nitrogen-free culture (NFC) medium (10.0 g/L mannitol, 0.2 g/L KH2PO4, 0.2 g/L NaCl, 0.2 g/L MgSO4• 7H2O, 0.2 g/L CaSO4• 2H2O, 5.0 g/L CaCO3, 20.0 g/L agar, pH 7.2) and Ashby medium (0.2 g/L KH2PO4, 0.2 g/L MgSO4, 0.2 g/L NaCl, 5.0 g/L CaCO3, 10.0 g/L mannitol, 0.1 g/L CaSO4, 15.0 g/L agar, pH 7.0). Strains exhibiting growth on both of the media were likely to utilize nitrogen for its growth through fixation of atmospheric nitrogen (Sen and Sen, 1965).
For assessment of IAA synthesis, bacterial isolates were inoculated in tubes containing 8 mL of King’ s B medium (20 g/L peptone, 10 g/L glycerol, 1.5 g/L K2HPO4, 1.5 g/L MgSO4, pH 7.2) and kept in an incubated shaker (120 rpm, 5 d, 30º C). The tubes containing the culture broth were then centrifuged at 12, 000 rpm for 5 min. Equimolar concentration of Salkowski reagent (1 mL 0.5M FeCl3 dissolved in 50 mL 35% HClO4) was added to 1.5 mL of supernatant. The mixture was incubated for 30 min and the absorbance was measured in a spectrophotometer at 530 nm (Giassi et al., 2016). Medium without bacterial suspension was used as a control. All experiments were done in triplicate.
Chrome azurol S (CAS) agar medium (Alexander and Zuberer, 1991) was prepared to measure the bacterial siderophore production. Preparation of the CAS agar involved mixing of four separate sterilized solutions: Solution 1 (Fe-CAS indicator), Solution 2 (piperazine-N, Nʹ -bis(2-ethanesulphonic acid) or PIPES buffer solution), Solution 3 (Basal medium) and Solution 4 (10%, w/v casamino acids). All the solutions were sterilized separately, cooled to 50º C and mixed with sufficient stirring to prevent formation of bubbles in the order as reported by Alexander and Zubere (1991). The bacterial isolates were inoculated on CAS agar and incubated at 28º C for 10 days. Formation of orange-color halo around the bacteria colony was considered as positive result for siderophore production.
The fungal causative agents of Verticillium wilt, i.e., Verticillium dahlia strain 991 and V. dahlia strain 7 were collected by Xinjiang Agricultural University, China, while Alternaria alternata (causative agent of leaf blight) was isolated by Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, China. The three fungi were maintained on potato dextrose agar (PDA) at room temperature for antagonism assay. The pathogenicity of these fungi was verified through Koch’ s postulates (Byrd and Segre, 2016).
Antagonism against these fungal pathogens was assayed by dual-culture in-vitro method. Fungal disc (6 mm in diameter) containing 5-day old culture from PDA plates was placed at the center of a fresh PDA plate. Four bacterial cultures grown on ISP 2 agar were then streaked at the corner of the PDA plates, 3 cm away from the fungal disc. Plates without the bacterial isolates served as controls. All plates were incubated at 28º C until the fungal mycelia in control plates reached the edge of the plates. Colony growth inhibition (%) was calculated by using the formula: I=(R0-Ri)/R0× 100%, where R0 is the radial growth of pathogen in control plates (measured in mm), and Ri is the radial growth of pathogen in test plates (mm). All experiments were done in triplicate.
No microbial growth was observed after 15 days of incubation at 30º C on ISP2 agar where the water used in the final rinse of surface-sterilization was plated. This indicated that the 3-step surface sterilization protocol was effective in inhibiting the growth of the epiphytic bacteria. The subsequent bacteria obtained during the isolation were, therefore, considered to be true endophytes.
3.1.1 Composition of endophytic bacteria
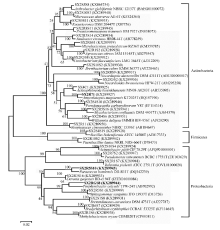
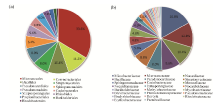
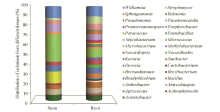
A total of 125 endophytes were isolated from surface sterilized tissues of F. sinkiangensis. Characterization by 16S rRNA gene sequencing indicated that they belong to bacteria of 3 phyla, 13 orders, 23 classes, 29 genera, and 58 species. The phylum Actinobacteria accounted for 88 bacteria isolated from F. sinkiangensis. The remaining isolates were represented by the phyla Proteobacteria and Firmicutes (28 and 13 isolates, respectively) (Fig. 1). The most prevalent endophytic bacteria in F. sinkiangensis was the order Micrococcales, representing 33.6% of the total isolates. The most predominantly isolated classes among these isolates were Microbacteriaceae, Micrococcaceae and Nocardiaceae, representing 16.8%, 12.8% and 10.4%, respectively (Fig. 2).
3.1.2 Putative novel species
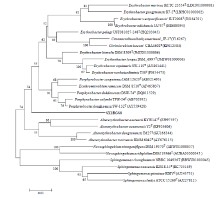
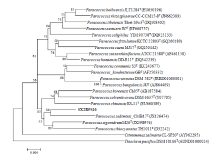
Strain SX2RGS8 exhibited 95.2% 16S rRNA gene sequence similarity with Porphyrobacter colymbi TPW-24T. While strain SX2R5S10 showed 97.6% 16S rRNA gene sequence similarity with Paracoccus homiensis DD-R11T, strain SX2R71 exhibited 97.8% 16S rRNA gene sequence similarity with Amycolatopsis magusensis KT2025T. Based on the consideration of Kim et al. (2014), it is likely that strains SX2RGS8, SX2R5S10 and SX2R71 are potential new members of the genera Porphyrobacter, Paracoccus and Amycolatopsis, respectively (Figs. 3-5).
3.1.3 Tissue-specificity of endophytes
The abundance of endophytic bacteria in root tissues was apparently higher than in stem. For instance, the distributions of the genera Bacillus, Kocuria, Nocardiopsis, Streptomyces, Sphingomonas and Williamsiawere higher in roots than in stems, while the genus Microbacterium was more abundant in stems than in roots. Sixteen rare genera Actinophytocola, Agrococcus, Amycolatopsis, Bradyrhizobium, Curtobacterium, Devosia, Janibacter, Nocardioides, Methylobacterium, Mycobacterium, Paenibacillus, Paracoccus, Porphyrobacter, Promicromonospora, Pseudomonas and Ralstonia representing 20 isolates were isolated only in root tissue during the study. Thirteen genera Acinetobacter, Arthrobacter, Bacillus, Brevibacterium, Brevundimonas, Kocuria, Microbacterium, Micrococcus, Nocardiopsis, Pseudonocardia, Sphingomonas, Streptomyces and Williamsia were isolated from both the root and stem tissues. A total of 27 genera (representing 93.1% of all genera obtained) occurred in roots, but only 13 genera (representing 44.8% of all genera) were isolated from stems (Fig. 6).
3.2.1 Phosphate solubilization
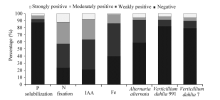
Of the total endophytic bacteria isolated, only 12.11% were observed to be positive for phosphate solubilization test (Fig. 7). Of these strains, 3.0% showed strong, 4.5% moderate and 4.5% weak phosphate-solubilizing activities. These strains were restricted to the genera Acinetobacter, Microbacterium and Ralstonia (Table 1).
 | Fig. 7 Distribution of positive strains tested for growth promotion traits; IAA, indole-3-acetic acid. |
| Table 1 Characteristics and growth promotion screening of some representative strains |
3.2.2 Nitrogen fixation
A large number of isolates (75.8%) showed positive growth on both NFC and Ashby media. The results are indicative of the potential of these strains for nitrogen fixation. Percentage of 12.1% of these strains, mostly Microbacterium spp., was strongly positive for growth on nitrogen-free media. While 30.3% of the total strains showed moderately activity, 33.3% exhibited weakly positive for putative nitrogen fixing ability (Fig. 7). Other genera exhibiting growth on nitrogen-free media include Acinetobacter, Bacillus, and Williamsia (Table 1).
3.2.3 IAA synthesis
Importantly, 79.4% of the tested isolates were capable of producing IAA (Fig. 7). Among the different genera, Bacillus strains such as S4S19, R7S5, 3A6, 3A8 and R10S11 produced the highest amount of IAA (Table 1).
3.2.4 Siderophore production
A total of 57.1% of the total isolates were found to produce siderophore. The frequency of strains exhibiting strong activity was low (1.4%; Fig. 7). Strains S4S19 (Bacillus), 3A6 (Bacillus), R10S11 (Bacillus) and S4S17 (Microbacterium) exhibited moderate siderophore production (Table 1).
3.2.5 Antagonism assay
A total of 40.6% of the total isolates exhibited antagonistic activity against A. alternata. The frequencies of bioactive strains inhibiting V. dahlia 991 and V. dahlia 7 were, however, 17.2% and 20.2%, respectively. The strains 3A8 (Bacillus), S4S19 (Bacillus), R10S11 (Bacillus) and 3A6 (Bacillus) showed strong inhibitory action against all the three tested pathogenic fungi A. alternata, V. dahlia 991 and V. dahlia 7 (Table 1).
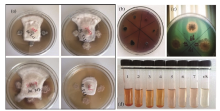
Our results found that strains of R4S5 (Acinetobacter), 3A6 (Bacillus), 3A8 (Bacillus) and R10S11 (Bacillus) were positive for nitrogen fixation, IAA synthesis and siderophore production assays in addition to antagonistic activity against A. alternata and V. dahlia 7. Few strains were positive for both PGP traits and biocontrol activity except for those four strains (Table 1). A representative picture illustrating the above activities is shown in Figure 8.
Endophytes have been isolated and identified from various cereal crops as well as from medicinal plants (Liu et al., 2016). These microorganisms are known to play an important role in ecological adaptation of the host plant. They may act to facilitate plant fitness through production of phytohormones or to provide protection through production of biologically active metabolites (Bloemberg and Lugtenberg, 2001; Nimaichand et al., 2016). Unlike in other areas, plants in arid and desert areas often face prolonged abiotic stresses such as water limitation and salt accumulation. Such plants often tend to select bacteria endowed with growth promoting and stress resistance traits for its symbiotic association (Marasco et al., 2012; Soussi et al., 2016; Asaf et al., 2017). It is therefore necessary to understand the diverse population of endophytic bacteria associated with plants especially those in arid region to provide a greater insight into the plant-endophyte interactions, bioactivity, and its ecological role. This study was conducted with the aim to understand the distribution and bioactivity of endophytic bacteria from F. sinkiangensis found in arid land of Shihezi, Xinjiang. During the present study, a total of 125 isolates belonging to 3 phyla, 13 orders, 23 families, and 29 genera were isolated from surface-sterilized root and stem tissues of the plant. In a similar study conducted by our group on another Ferulaplant samples collected from Hoboksar Mongol Autonomous County, Xinjiang (Liu et al., 2016), 170 endophytic bacteria distributed into 3 phyla, 15 orders, 20 families and 27 genera were isolated. Kaplan and co-workers isolated 31 endophytic strains belonging to 3 phyla, 20 genera from roots of two dominant shrubs found in Negev Desert (Kaplan et al., 2013). These results confirm the rich microbial diversity in plants grown in arid lands.
During the present study, root tissues were found to be a more suitable host for endophytic bacteria (52.8% of all isolates) as compared with stem (47.2%). This is further illustrated by the fact that a total of 27 genera were isolated from roots as compared with 13 genera from stems. Similar results were also found during endophytic studies in Azadirachia indica, Cucumis sativus, Ferula songorica, Maytenus austroyunnanensisand Oryza sativa (Mano and Morisaki, 2008; Verma et al., 2009; Qin et al., 2012; Liu et al., 2016). This phenomenon may be associated with the proposition that rhizospheric bacteria were laterally transferred towards the internal plant tissues (Santoyo et al., 2016). In addition, bacteria residing in the rhizosphere might also have the potential to colonize the plant roots, as living within plant tissues can provide an opportunity to be in contact with the plant’ s cells and thereby exert a direct beneficial effect.
Microbial assemblages and their species distributions in specific environment are determined by the environmental parameters (Jose and Jebakumar, 2014; Sibanda et al., 2017). Microorganisms isolated from unusual environments such as endophytes of arid plants were most likely unique in nature. In a recent study, two novel bacteria of higher bacterial hierarchy (of the status novel order) were isolated from arid soils in Xinjiang (Zhang et al., 2016a, b). Bacillus shacheensis strain HNA-14T, a moderately halophilic bacterium, was isolated from a soil sample in Shache County, Xinjiang (Lei et al., 2014). And another novel halophilic bacterium Okibacterium endophyticum was isolated from roots of halophyte Salsola affinis collected from Xinjiang (Wang et al., 2015). The rich endophytic resources of arid regions in Xinjiang are further proved by the isolation of putative novel taxa in the current study. Based on the 16S rRNA gene sequences analysis, strains SX2RGS8, SX2R5S10 and SX2R71 isolated from root tissues of F. sinkiangensis could be new member of the genera Porphyrobacter, Paracoccus, and Amycolatopsis, respectively.
The plant growth-promoting activities of microorganisms can be observed by direct and indirect methods. Direct plant growth promotion requires production or supplementation of essential factors required for the growth of the plant through biological nitrogen fixation, solubilization of phosphates, siderophore production, and IAA synthesis. Among these factors, biological nitrogen fixation and solubilization of phosphates are important as these processes can convert the most readily but unutilizable nitrogen and phosphorus sources to accessible forms (Meunchang et al., 2006; Nimaichand et al., 2016). Nitrogen fixation by bacteria can be accessed directly by their ability to grow on nitrogen-free media or reduce acetylene, and indirectly through PCR amplification of nif genes (Gtari et al., 2012). In the current study, a large number of the endophytes (75.8%) were able to grow in nitrogen-free medium, which gives an indirect hint on its ability to fix nitrogen. However, additional experiment such as detection of nifH genes will be necessary to further confirm its capacity to fix nitrogen. Additionally, seven strains belonging to Microbacterium and Acinetobacter were positive for growth in nitrogen-free medium (nitrogen fixation) and phosphate solubilization activity.
Endophytes also play an important role in growth promotion by controlling and minimizing the deleterious effects of external factors such as fungal infections, water stress, etc. This, in turn, improves the overall health and fitness of the plant. One such example is the IAA synthesis by endophytes that promotes the growth of plant and, thereby, increases the yield (Patten and Glick, 1996; Aldesuquy et al., 1998; Pereira et al., 2012). Several reports indicated that the production of siderophores by beneficial microbes is perceived as a means of biological control of phytopathogens especially effective in controlling fungal pathogens (Schippers et al., 1987). Additionally, microbes depicting different modes of action have a higher success rate in suppressing disease, and such microbes are potential good candidates as biocontrol agent (Palaniyandi et al., 2013). In this study, 79.4% and 57.1% of the total isolates were capable of producing IAA and siderophore, respectively. And, 40.6% of the strains inhibit the growth of A. alternata, 17.2% and 20.2% strains were positive for antagonism against V. dahlia 991 and V. dahlia 7, respectively. Among these positive strains, four strains showed strong antagonistic activities against all the three pathogenic fungi. Similar results were also reported in halophytes from Xinjiang (Wang, 2015), Allium sativum from Shanxi (Cui et al., 2008), and citrus rootstocks from Brazil (Giassi et al., 2016).
The present study represents the first report on the study of distribution and bioactivity of endophytic bacteria from F. sinkiangensis. The study showed that the approach involving microbial cultivation along with 16S rRNA gene sequences analysis is a useful tool in exploring endophytic bacterial resources on different tissues. These results also provide a preliminary framework for exploring endophytic bacteria associated with endangered medicinal plant F. sinkiangensis as potential bioinoculants for agriculture application. In the light of this, our future work will be directed towards exploring more endophytic bacteria resources from arid and semi-arid environment and towards understanding the mechanisms of biocontrol and plant growth promotion with special emphasis on nitrogen fixation, phosphate solubilization, IAA synthesis, siderophore production and resistance to different pathogenic fungi.
This work was supported by the National Natural Science Foundation of China (U1403101, 31200008), the China Postdoctoral Science Foundation (2016M602566), the Visiting Scholar Grant of State Key Laboratory of Biocontrol, Sun Yat-Sen University (SKLBC14F02) and the West Light Foundation of the Chinese Academy of Sciences. The author LI Wenjun was also supported by Hundred Talents Program of the Chinese Academy of Sciences and Guangdong Province Higher Vocational Colleges & Schools Pearl River Scholar Funded Scheme (2014).
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|