Knowledge of soil respiration and the influencing factors in desert ecosystems is essential to understanding carbon dynamics and responses of biotic and abiotic processes in soils to climate change. In this study, soil respiration rate ( Rs) for three land-cover types (shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land) in the hinterland of the Taklimakan Desert was measured in May 2015 using an automated soil CO2 flux system. The effects of soil temperature ( Ts) and soil water content ( Ws) on Rs were also analyzed. The results showed that Rs values in shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land were all low and exhibited obvious diurnal fluctuations. The establishment of straw checkerboard barriers in sandy land had no significant effect on Rs, while the establishment of shelterbelts significantly increased Rs. Shifting sandy land and sandy land with straw checkerboard barriers were carbon sinks at night and early morning and were carbon sources in the daytime, while shelter forest land always acted as a carbon source during the whole day. The synergistic effect of Ts and Ws could better explain the diurnal dynamics in Rs than single factor. In shifting sandy land and sandy land with straw checkerboard barriers, Ws was identified as a limiting factor influencing the diurnal dynamics of Rs. Furthermore, a relatively strong hysteresis loop existed between Rs and Ts. In contrast, in shelter forest land, Rs was significantly influenced by Ts, and a relatively weaker hysteresis loop existed between Rs and Ws.
Soil is the main carbon pool in terrestrial ecosystems (Schlesinger and Andrews, 2000; Huang et al., 2007). The gross storage of organic carbon in soil is approximately 1395-2011 Pg (1 Pg=1015 g), accounting for ~67% of the total carbon amount in terrestrial ecosystems (Jenkinson et al., 1991; Jin et al., 2009). The exchange process of carbon dioxide (CO2) between the soil and atmosphere is referred to as soil respiration. Generally speaking, soil respiration includes four biotic processes (including plant root respiration, soil microbial respiration, soil animal respiration and soil organic matter decomposition) and many abiotic processes (such as chemical oxidation of carbonaceous matter, dissolution of carbonate, change of pressure gradients caused by wind and so on) (Singh and Gupta, 1977; Lou and Zhou, 2006; Jin et al., 2007; Feng et al., 2008). Soil respiration is the major route of carbon released from soil to atmosphere. At the global scale, the estimated amount of carbon released from soil to atmosphere is 50-75 Pg annually, which is higher than the net primary production (Jenkinson et al., 1991). Even slight variations of soil respiration can have far-reaching effects on the atmospheric CO2concentration, and could generate a positive feedback on climate change (Rustad et al., 2000; Qi et al., 2006; Gao et al., 2012).
Desert, an important terrestrial ecosystem, is characterized by extreme drought, higher surface albedo, sandy underlying surface, and frequent sand-dust weather (Cable et al., 2011). Although desert ecosystem is generally low in soil organic carbon content, it plays an important role in soil carbon cycle (Liu et al., 2015). There are some recent researches on soil respiration in desert areas (e.g., Sierra, 2012; Jia et al., 2013; Zhang et al., 2015). A pervious study showed that soil respiration rate (Rs) in desert areas is relatively high in fixed sandy land, relatively low in semi-fixed sandy and lowest in shifting sandy land (Li et al., 2008).Rs in desert areas is affected by many factors, such as biomass of the phyto-community, soil organic carbon content, soil total nitrogen, soil temperature (Ts), soil water content (Ws), etc. (Sierra, 2012). Ts and Ws are the two most important factors influencing Rs (Sierra, 2012) because Ts and Ws strongly modulate soil organic matter decomposition, root respiration, and microbial activity. It was reported that a significant hysteresis effect exists between Rs and Ts (Subke et al., 2003; Jia et al., 2013), and low levels of Ws could increase the degree of hysteresis (Phillips et al., 2011; Wang et al., 2013). Thus, Ws is of great importance in influencing Rs in desert ecosystems (Cable et al., 2011; Zhang et al., 2015). Recent studies proposed that desert ecosystems, which have long been neglected in the studies of global carbon budget, exhibit strong downward fluxes into the ground and therefore might be a significant carbon sink (Jasoni et al., 2005; Stone, 2008; Wohlfahrt et al., 2008; Ma et al., 2014). This may be due to that highly saline-alkali soil can promote chemical reactions in the presence of moisture, such as the dissolution of calcium carbonate that causes atmospheric CO2 to be absorbed by the soil (Stone, 2008; Xie et al., 2009; Ma et al., 2013, 2014). Thomas and Hoon (2010) pointed out that soil could absorb atmospheric CO2 with assistance of soil microorganisms. In addition, studies on the response of soil respiration to precipitation indicated that precipitation in arid areas could strongly increase Rs (Huang et al., 2007; Sponseller, 2007; Zhao et al., 2015). Soil respiration in desert areas is generally weak, thus it is easily ignored in related studies, limiting the needed in-depth understanding of soil respiration (Chen and Tian, 2005; Cable et al., 2011).
Desert covers an area of 0.7× 106 km2 in China, with Taklimakan Desert being the largest one (accounting for about half of this area). In recent years, with the increase of human activities, a large number of straw checkerboard barriers and shelter forest belts were set up in the hinterland of the Taklimakan Desert to protect oil transportation and gas production from damage of drifting sands. As a result, the ground surface cover mainly consisting of continuous shifting sands was gradually changed and the regional biogeochemical cycles including carbon cycle were consequently affected.
In this study, Rs, Ts and Ws at 5-cm depth were synchronously monitored for three land-cover types (shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land) in the hinterland of the Taklimakan Desert. The objectives of this study were (1) to compare the diurnal dynamics of Rs for the three land-cover types; (2) to discuss the effects of Ts and Ws on Rs; and (3) to explore the hysteresis effects between Rs and Ts and between Rs and Ws.
This study was conducted in the vicinity of the Taklimakan Desert Atmosphere and Environment Observing and Experimental Station (38° 58′ N, 83° 39′ E; 1099 m a.s.l.), and the study area is situated in the Tazhong area (the hinterland) of the Taklimakan Desert, Xinjiang, Northwest China (Fig. 1). The distance from the station to the edge of the desert is about 229 km. The study area is characterized by a warm-temperate arid desert climate with low precipitation (mean annual precipitation of 25.9 mm) and high potential evaporation (annual potential evaporation of 3812.3 mm). It should be noted that most of annual total precipitation occurs from May to August. The annual mean air temperature is 12.1° C, the maximum air temperature ranges from 40.0° C to 46.0° C, and the minimum air temperature ranges from -32.6° C to -20.0° C. Natural vegetation coverage in the study area is relatively low. The ground surface is mainly covered by drifting sands. The prevailing wind comes from east and the annual mean wind velocity is 2.3 m/s. The mean occurrence of weather events with floating dust and blowing sand is more than 157 d/a, and the mean sandstorm occurrence is 16 d/a. The landscapes in the study area are mainly dominated by the inter-dune corridors and the linear and longitudinal dunes (direction of NNE-SSW or NE-SW; relative height of 40-50 m).
Three land-cover types were selected: shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land (Fig. 2). For shifting sandy land, there was almost no vegetation at all. For sandy land with straw checkerboard barriers, the straw checkerboard barriers were made of reed that was placed (with half buried in the sand) in the shape of a checkerboard pattern (an area of 1.0 m× 1.0 m for each checkerboard). The straw checkerboard barriers were installed in 2001. For shelter forest land, the shelter forest belts were dominated by Haloxylon ammodendron, Calligonum arborescensand Tamarix ramosissima planted in 2002. Since sand-dust weather occurs frequently in the hinterland of the Taklimakan Desert (i.e., Tazhong area), the straw checkerboard barriers, and shelter forest belts play important roles in wind prevention and sand stabilization. In May, the shelter forest experiences rapid leaf growth and canopy expansion, thus the protection effect is especially obvious.
For each land-cover type, three sampling plots with each area of 10 m× 10 m were randomly selected. Soil respiration rate (Rs) was measured with an automated soil CO2 flux system (Model LI-8100A fitted with a LI-8150 multiplexer, LI-COR, Nebraska, USA) during the periods of 1-8 May and 12-14 May, 2015. For each sampling plot, three polyvinyl chloride (PVC) cylindrical soil collars (cross section of 371.8 cm2 and height of 10 cm) were separately embedded into the soil to a depth of 8 cm. It should be noted that these PVC collars were installed 3 days before the measurements to avoid the influences of installation-resulted disturbance on Rs. After 72 h, Rs can be returned to the initial level before the installation of PVC collars. During the process of instrument parameter setting, the measurement time was prolonged to ensure the measurement accuracy. The stabilization time before gas extraction from the air chamber was set as 60 s, and the measurement time of gas extraction and the stabilization time after measurements were set as 150 and 30 s, respectively. For each chamber, measurements were conducted at 30-min intervals. Meanwhile, for each land-cover type, soil temperature (Ts) and soil water content (Ws) at 5-cm depth were measured near the chambers using the 8150-203 soil temperature sensor and EC-5 soil moisture sensor (LI-COR, Nebraska, USA), respectively. After the measurements ofRs, the 0-10-cm topsoil inside the PVC collars was collected to analyze its physical and chemical properties. Soil total salt, organic carbon, total nitrogen, and pH were measured by the residue-drying method, potassium dichromate-volumetric method, AA3 continuous flow analytical system and pH-3C pH Meter (Bao, 2000), respectively. Soil physical and chemical properties in the topsoil layer for the three land-cover types are presented in Table 1.
 | Fig. 2 Layout of the three land-cover types and soil CO2 flux monitoring equipment. (a), shifting sandy land; (b), sandy land with straw checkerboard barriers; (c), shelter forest land. |
| Table 1 Soil physical and chemical properties in the 0-10-cm topsoil layer in shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land |
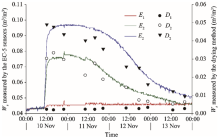
It should be noted that under low Ws conditions in the study area, the EC-5 soil moisture sensors matched by an automatic soil CO2 flux system can easily cause measurement errors. Therefore, it is necessary to correct the Ws measurements (using the EC-5 soil moisture sensors) by the traditional drying method. In this study, we conducted a supplementary experiment in November 2016 to correct the Ws measured using the EC-5 soil moisture sensors in the field. Specifically, samples of topsoil layer (0-10 cm) from shifting sandy land were divided into three portions and were dissolved into 0, 180, and 270 mL of distilled water, respectively. After efficient stirring, they were moved into three same cylindrical boxes to form three soil moisture gradients. Then, the three cylindrical boxes were placed into an artificial climatic chest (Model HP1500GS, Ruihua Inc., Wuhan, China), with air temperature maintaining at 30° C, air humidity at 20%, and illumination at level 2. Three EC-5 soil moisture sensors were then embedded into the boxes at a burial depth of 5 cm, and continuously recorded the Ws changes. Meanwhile, Ws was measured by the traditional drying method in the same layer, i.e., 5-cm depth. The results are shown in Figure 3. The results of two measuring methods exhibited a certain similarity. It should be noted that although the EC-5 soil moisture sensor can reflect variations in Ws with time, a certain level of discrepancy in values between the two methods still exists: the lower the Ws, the larger the discrepancy. In the treatment of added 0 mL of distilled water, the average Ws value measured by the EC-5 soil moisture sensors was 20 times that by the drying method. Thus, 20 was regarded as the correction coefficient of the Ws measured by the EC-5 soil moisture sensors in the field.
In this study, statistical analysis was performed using SPSS 16.0 and Sigmaplot 12.5. Correlation analysis and partial correlation analysis were used to examine the relationships between Rs (µ mol/(m2• s)), Ts (° C) and Ws (m3/m3) at 5-cm soil depth for the selected three land-cover types. Regression analysis was used to determine the effects of Ts and Ws on Rs. The following three types of non-linear functions were used to analyze the synergistic effect of Ts and Ws on Rs:
Rs=a+b× Ts+c× Ws, (1)
Rs=a+b× Ts+c× Ws+d× Ts× Ws, (2)
Rs=a× Tsb × Wsc, (3)
where, a, also known as basal respiration rate, is the soil respiration rate when Ts and Ws are both equal to 0 (µ mol/(m2• s); b and c are reaction coefficients of Ts and Ws, respectively; and d is the coefficient of internal interaction between Ts and Ws.
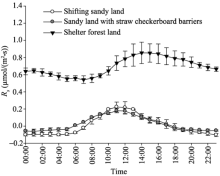
Rs in shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land all displayed obvious diurnal fluctuations. Rs in shifting sandy land and sandy land with straw checkerboard barriers exhibited similar diurnal dynamics (unimodal curves) with low values (Fig. 4). In comparison, Rs values in shelter forest land were high and the diurnal dynamic was different from those in the other two land-cover types. Specifically, diurnalRs values in shifting sandy land and sandy land with straw checkerboard barriers ranged from -0.10 to 0.22 µ mol/(m2• s) (average of 8.9× 10-3µ mol/(m2• s)) and from -0.06 to 0.18 µ mol/(m2• s) (average of 24.4× 10-3 µ mol/(m2• s)), respectively. DiurnalRs values in shelter forest land ranged from 0.54 to 0.85 µ mol/(m2• s), with an average of 0.693 µ mol/(m2• s), being 77.9 and 28.4 times larger than those in shifting sandy land and sandy land with straw checkerboard barriers, respectively. The diurnalRs values in shifting sandy land were consistently negative from 17:00 (Beijing time) on the preceding day to 08:00 on the succeeding day and the diurnalRs values in sandy land with straw checkerboard barriers were consistently negative from 17:00 on the preceding day to 06:00 on the succeeding day, indicating that soil absorbed CO2 from the atmosphere during this negative-value period. After this negative-value period, Rs values became positive with the maximum values both occurred at 11:00 (0.22 and 0.18 µ mol/(m2• s), respectively) and then decreased rapidly. For shelter forest land, diurnalRs values were all positive throughout the day, suggesting that this land-cover type always maintained in a state of soil CO2 release. The diurnal maximum of Rs for shelter forest land was delayed by 3 h with respect to those for the other two land-cover types. The aforementioned results show that, in the hinterland of the Taklimakan Desert, the establishment of straw checkerboard barriers in sandy land had no significant influence on Rs, while the establishment of shelter belts significantly increased Rs.
Correlation analyses and regression analyses between Rs and Ts in shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land are shown in Figure 5. A significant positive correlation was found between Rs and Ts in shelter forest land (r=0.983, P< 0.001; Fig. 5c). The correlation between Rs and Ts in shifting sandy land was lower (r=0.757, P< 0.001) than that in shelter forest land. The maximum Rs appeared earlier than the maximum Ts, presenting a clear hysteresis phenomenon (Fig. 5a). For the three land-cover types, the hysteresis phenomenon in sandy land with straw checkerboard barriers was the most obvious. In addition, there was a negative correlation between Rs and Ts (r= -0.225, P=0.291) for in sandy land with straw checkerboard barriers (Fig. 5b). Using artificial elimination of the hysteresis in shifting sandy land and sandy land with straw checkerboard barriers (i.e., advancing Ts by 2 and 3 h, respectively), the correlation coefficients between Rs and Ts increased to 0.957 (P< 0.001) and 0.971 (P< 0.001), respectively.
For shelter forest land, a linear regression model was best for fitting the relationship between Rs and Ts (R2=0.849, P< 0.0001; Fig. 5f). For shifting sandy land and sandy land with straw checkerboard barriers, however, the linear relationship between Rs and Ts was weak (Figs. 5d and e, respectively). An obvious clockwise loop was found when plotting Rs against Ts, especially for sandy land with straw checkerboard barriers. In the clockwise loop, Rs values in the increasing stage were higher than Tsvalues in the decreasing stage. According to the distribution of the scatter points, the segmentation fitting was carried out with 05:00 and 13:00 as the boundaries. The fitting results are listed in Table 2. In general, from shelter forest land through shifting sandy land and to sandy land with straw checkerboard barriers, the consistency between Rs and Ts gradually decreased. However, with respect to Rs, the hysteresis effect of Ts was gradually intensified, that is, the ‘ hysteresis loop’ was most obvious in sandy land with straw checkerboard barriers.
| Table 2 Regression equations between Rs (soil respiration rate) and Ts (soil temperature) at 5-cm depth for shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land |
Ws is another significant factor affecting Rs. Correlation analyses and regression analyses between Rs and Ws are shown in Figure 6. There were obvious diurnal fluctuations for both Rs and Ws (Figs. 6a-c). Generally speaking, the shifting sandy land had the best consistency between Rs and Ws, showing a significant positive correlation (r=0.935, P< 0.001). The correlation between Rs and Ws in shifting sandy land with straw checkerboard barriers was slightly lower (r=0.901, P< 0.001) than that in shifting sandy land. In shelter forest land, Rs and Ws also presented a hysteresis phenomenon but was different from the hysteresis between Rs and Ts. That is, the maximum Rs appeared later than the maximum Ws (r=0.727, P< 0.001). After eliminating the hysteresis between Rs and Ws, the coefficient was increased to 0.959 (P< 0.001). All in all, even a slight variation in Ws could result in a considerable change in Rs.
For shifting sandy land and sandy land with straw checkerboard barriers, the exponential equation could better describe the relationship between Rs and Ws (Figs. 6d and e; Table 3), with R2 being 0.822 (P< 0.001) and 0.691 (P< 0.001), respectively. For shelter forest land, the regression distribution of Rs and Ws showed a relatively weak circular hysteresis (Fig. 6f). This hysteresis loop was counterclockwise, indicating that the phase of Rs lagged behind that of Ws.
| Table 3 Regression equations betweenRs and Ws (soil water content) at 5-cm depth for shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land |
In the model that combines Ts and Ws, Equations 1-3 (in Section 2.3) were all used to analyze the synergistic effect of Ts and Ws on Rs. As shown in Table 4, these equations could well describe the responses of Rs to variations in Ts and Ws for all the three land-cover types (P< 0.01), with an exception of Equation 3, which failed to explain the synergistic effect of Ts and Ws on Rs in shifting sandy land and sandy land with straw checkerboard barriers. The result indicates that Rs in the study area was affected by Ts and Ws together. In general, Equations 1 and 2 could better explain the diurnal dynamics of Rs for the three land-cover types. The combination of Ts and Ws can explain more than 88% of the total variation of Rs.
| Table 4 Multiple regression equations and curve fitting analyses for Rs with Ts and Wsfor shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land |
Rs values in shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land in the hinterland of the Taklimakan Desert were significantly lower than those in the other land-cover types, e.g., deciduous broadleaf forest, evergreen needle leaf forest, grassland, and woodland/savanna (Hibbard et al., 2005). Lower Rs values in the study area were mainly caused by extreme climate conditions (low precipitation, high evaporation, and frequent sand-wind weather), relatively few soil microorganisms and animals, and scarce vegetation (low root respiration). Generally speaking, the establishment of straw checkerboard barriers in sandy land in the hinterland of the Taklimakan Desert had little effect on Rs, while the establishment of shelterbelts significantly increased Rs. The establishment of shelterbelts not only increased the plant root respiration, but also changed the soil physical and chemical properties, providing beneficial conditions to the activities of soil microorganisms and animals and resulting in the increase of Rs. Similar to the results in other studies (Huang et al., 2007; Zhang et al., 2007; Wang et al., 2013), Rs values for the three land-cover types all showed obvious diurnal fluctuations. It is noteworthy that the phase of diurnal change in Rs for shelter forest land lagged behind than those for shifting sandy land and sandy land with straw checkerboard barriers. Both Ts and Ws showed hysteresis with Rs in shelter forest land. This may be due to that the increased vegetation cover reduced the sensitivity of Ts and Ws to the variations in external environmental conditions.
For shifting sandy land and sandy land with straw checkerboard barriers, soil absorbed CO2 from the atmosphere at night and early morning and released CO2 into the atmosphere during the daytime, being consistent with those previously reported. For examples, the saline-alkali soil of the Gurbantunggut Desert in northern Xinjiang of China was subjected to potential chemical reactions with the action of soil moisture at night and early morning and then calcium carbonate in the soil was dissolved, promoting soil CO2absorption (Stone, 2008; Xie et al., 2009). By sterilization control experiment, Wang et al. (2013a, b) illustrated that soil CO2absorption was a comprehensive reflection of soil organic and inorganic CO2fluxes and that the absorption of inorganic carbon at night was one of the main reasons for forming carbon sink in the deserts. They also found that the saline-alkali soil with high pH value and low temperature could promote soil CO2absorption. Similarly, Ma et al. (2013) showed that in the Gurbantunggut Desert, inorganic processes could cause the negative soil CO2 flux, forming a carbon sink. Due to the fact that soil is quite dry in the desert ecosystems, all soil CO2 fluxes may originate from inorganic processes (which are affected by Ts). Furthermore, soil pH controls the amplitude of variation of soil CO2 flux to some extent. The soil in the hinterland of the Taklimakan Desert exhibited high salinity and high alkali characteristics, with soil pH in the range of 8.66-9.54 (average of 9.22) and total salinity content in the range of 0.27‰ -1.86‰ (average of 0.78‰ ). Therefore, we speculate that under high soil salinity and alkali conditions, soil inorganic carbon process is one of the main reasons to form carbon sink in the Taklimakan Desert.
It was previously reported that Ts, Ws and the combination of Ts and Ws are the main driving factors influencing Rs in arid and semi-arid regions (e.g., Zhang et al., 2007; Wang et al., 2012). In the hinterland of the Taklimakan Desert, a robust linear relationship between Rs and Tswas found in shelter forest land, and a segmentation fitting was observed in shifting sandy land and sandy land with straw checkerboard barriers, being similar to those for the same land-use types in the northern margin of the Taklimakan Desert (Yang et al., 2015) and being dissimilar to the results for other land-use types (Huang et al., 2007; Zhang et al., 2007). However, all these observations indicated that Ts was an important factor influencing Rs, and the responses of Rs to Ts were different in different land-use types. In desert ecosystems, Ws is another major influencing factor on soil respiration processes (Oechel et al., 2000; Zhou et al., 2009). In the hinterland of the Taklimakan Desert, exponential relationships between Rs and Wswere found in shifting sandy land and sandy land with straw checkerboard barriers, while hysteresis phenomenon was observed in shelter forest land. The synergistic effect of Ts and Ws could better explain the diurnal dynamics of Rs.
In this study, regression analyses of Rs and environmental factors (Tsand Ws) showed that obvious circular hysteresis loop existed between Rs and environmental factors. The hysteresis loop was clockwise when the phase of Rs was advanced relative to the environmental factor. The hysteresis loop was counterclockwise when the phase of Rs was lagged behind the environmental factor. This phenomenon was also found in the Xiaotang area of the Taklimakan Desert (Yang et al., 2015) and the Yanchi area of the Mu Us Desert (Wang et al., 2013). According to the partial correlation analysis of the relationships between Rs and Ts and between Rs and Ws, Rs and Ts was negatively correlated when Ws was the control variable (Table 5). In contrast, Rs and Ws was positively correlated when Ts was the control variable. Ws in the hinterland of the Taklimakan Desert was low and was likely the limiting factor of soil respiration in shifting sandy land and sandy land with straw checkerboard barriers. The response of Rs to the diurnal variations in Ws was more direct and sensitive, and the corresponding regression relationship exhibited an exponential function. For shelter forest land, plant root was the main contributor to soil respiration, and therefore the diurnal variations of Ws in short periods did not significantly affect Rs. Accordingly, the hysteresis loop between Rs and Ws was also relatively vague. The response of Rs to the diurnal variations of Ts was more direct in shelter forest land.
| Table 5 Partial correlation analyses of Rs, Ts and Ws for shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land |
In the hinterland of the Taklimakan Desert, we found that there were relatively obvious diurnal dynamics of Rs, Ts and Ws, and also robust regression relationships between Rs and Ts and between Rs and Ws at 5-cm depth for shifting sandy land, sandy land with straw checkerboard barriers, and shelter forest land. The synergistic effect of Ts and Ws could better explain the diurnal dynamics of Rs. The establishment of straw checkerboard barriers in sandy land had no significant effect on Rs, while the establishment of shelterbelts significantly increased Rs and slowed or smoothed the responses of Rs to the variations in Tsand Ws. Shifting sandy land and sandy land with straw checkerboard barriers acted as carbon sinks at night and early morning and acted as carbon sources in the daytime. However, shelter forest land acted as a carbon source during whole day because Rs was always in a state of soil CO2 release.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (41175140) and the National Department of Public Benefit (Meteorology) Research Foundation (GYHY201306066).
The authors have declared that no competing interests exist.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 20 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|
| 35 |
|
| 36 |
|
| 37 |
|
| 38 |
|
| 39 |
|