Carex brunnescens (Pers.), a typical clonal species, is considered to be the only herb found to date that can develop on sand dunes in Maqu alpine region of northwestern China. However, the characteristics that C. brunnescens resists to harsh alpine environment have not been documented. In this study, we conducted a field investigation to determine the morphological, reproductive, and sand-fixing characteristics of C. brunnescens. Concomitantly, we transplanted the belowground rhizomes of C. brunnescens to sand dunes and compared the abilities to restore degraded alpine meadows among sand dunes that had no further treatment (SD+N), sand dunes that had straw checkerboard technique but no transplanted rhizomes of C. brunnescens (SD+SCT), and sand dunes that had both SCT and transplanted rhizomes of C. brunnescens (SD+SCT+P). We found that belowground vertical rhizomes and horizontal rhizomes (including branching rhizomes and main rhizomes) of C. brunnescens were highly developed and that population reproduction was dominated by horizontal rhizomes. C. brunnescens exhibited a significant sand-fixation effect under following conditions: population density was 145-156 ramets/m2, vegetation cover was 31.2%-39.3%, total length of belowground rhizomes was 11,223 cm/m2, total length of belowground first-order roots was 9161-10,524 cm/m2, fresh weight of aboveground part was 198.5-212.6 g/m2, and fresh weight of belowground part was 578.8-612.4 g/m2. It should be particularly noted that SD+SCT+P treatment (sand dunes that had both straw checkerboard technique and transplanted rhizomes of C. brunnescens) was the best and SD+N (sand dunes that had no further treatment) was the worst in terms of following biotic indicators: total number of reproductive ramets, total number of belowground rhizomes, and fresh weight of aboveground and belowground parts of C. brunnescens, contents of soil organic carbon, available nitrogen, microbial biomass carbon, and microbial biomass nitrogen. It implies that applying SCT in sand dunes and transplanting belowground rhizomes to sand dunes with SCT could improve both soil fertility and growth of C. brunnescens. These results suggest that the SCT-promoted high reproductive abilities of belowground rhizomes of C. brunnescens can successfully facilitate the establishment of ramets and can thus be an effective strategy to restore degraded vegetation in Maqu alpine region of northwestern China.
Desertification is a process of land degradation resulting from climatic changes and human activities in arid and semi-arid areas. Desertification involves severe vegetation degradation and intensified wind and water erosions (Zhao et al., 2009). In particular, desertification caused by wind erosion poses a severe threat to agricultural productivity and ecological environment (Gomes et al., 2003; Lian et al., 2013). Even worse, regional political instability and social insecurity have also been linked to desertification (Meadows and Hoffman, 2002).
Sand burial and wind erosion are two of the most serious abiotic stresses on plant growth in many arid and semi-arid areas (Li et al., 2010a; Luo and Zhao, 2015). Deep sand burial can significantly inhibit plant growth and succession. However, stimulating effects on plant growth were also observed when plants were subjected to relatively shallow sand burial (Dong et al., 2000; Dech and Maun, 2006; Zhao et al., 2007; Jiang et al., 2016). Wind erosion can render soil infertile through the removal of nutrient-rich surface soil and a progressive decrease in vegetation cover (Yu et al., 2008; Li et al., 2010a, b). Clonal plants, the dominant plant type growing in sand dune environments, have developed multiple adaptive strategies to resist sand burial and wind erosion (Roiloa et al., 2014; Luo and Zhao, 2015). Clonal plants can generate new offspring ramets connected with parent plants. Relative to parent plants, the new ramets could achieve clonal expansion horizontally and thus enter into different habitats (Ye and Dong, 2011; Kang et al., 2016a). Clonal integration is considered to be one of the most important strategies for the survival of clonal plants because it allows buried ramets to access to water and nutrients via non-buried ramets, thus ensuring plant survival and reproduction in the environments that are prone to sand burial and wind erosion (Amsberry et al., 2000; Xu et al., 2010; Luo et al., 2015).
Maqu is one of the most important alpine meadows at the eastern edge of the Tibetan Plateau in northwestern China. The area is known as “ the kidney of the Yellow River” because of its vital function in conserving soil and water in the upper reaches of the Yellow River (Kang et al., 2015). However, long-term over-exploitation of alpine meadow associated with rapid population increases, environmentally-unfriendly means of agricultural production, overgrazing by livestock, mining, and urbanization inevitably triggered severe desertification in this region (Qi et al., 2006; Wei et al., 2010). The situation has been worsened by human alteration of river channels and also by human withdrawal of groundwater in the Yellow River (Wang et al., 2001, 2005). Consequently, the Maqu alpine region has risk to become a main source of sandstorms in China if effective control measures are not implemented (Wang et al., 2008).
Carex brunnescens(Pers.) is considered to be the only herb species found to date that can develop on sand dunes in Maqu alpine region (Kang et al., 2016a, b; Ma et al., 2017). This species is a typical clonal plant that is reproduces by forming strong belowground horizontal rhizomes. Due to low seed germination rate, C. brunnescens rarely reproduces sexually even in high-precipitation months or in wet years (Wang et al., 2013; Zhu et al., 2013). C. brunnescens is highly tolerant to wind erosion and also to sand burial because of its rapid growth and regeneration capacities of rhizomes, basal meristem and belowground storage organs (Kang et al., 2016a, b). Recent studies showed that C. brunnescens population can quickly invade and multiply in a new sand habitat with asexual reproduction by belowground rhizomes and thus can stabilize sand dunes. In favored topographical conditions, it can even form rare “ herb dunes” (Kang et al., 2016a, b; Ma et al., 2017). However, studies focusing on how the C. brunnescens resists sand burial and wind erosion are relatively scarce. Further, although the application of C. brunnescens in stabilizing sand dunes is of great interest, the feasibility of combining specific herbaceous plants with other means for enhancing wind protection and sand fixation effect has not been explored. The straw checkerboard technique (SCT) was devised at the Shapotou Desert Research Station, Chinese Academy of Sciences in 1957 (SDRS, 1986) and has been widely used since for stabilizing sand dunes in arid and semi-arid regions of China (Qiu et al., 2004; Ma et al., 2015). SCT refers to a simple mean that straws of wheat, or rice, or reed, or others were placed (with half buried in the sand) in the shape of checkerboard barriers. The straw checkerboard barriers can decrease wind velocity near the ground surface and thus diminish wind erosion of soils. It not only stabilizes the surface of sand dunes but also improves soil qualities through organic inputs of decomposed straw, dead branches and fallen leaves (Jiang et al., 2008; Chen et al., 2013; Kang et al., 2015). In regions where the annual precipitation is very low, some plants such as shrubs of Caragana microphyla Lam., Hedysarum fruticosum Pall. andSalix oritrepha Sohneider and herb of Agropyron cristatum L. can be planted to further improve the windbreak and sand dune fixation effects (Qiu et al., 2004; Jiang et al., 2008; Kang et al., 2013, 2015).
In this study, we selected the typical clonal herbC. brunnescens to determine its morphological, reproductive, and sand-fixing characteristics through a field investigation in a sand dune environment prone to wind erosion and sand burial. Further, we attempted to analyze the influences of belowground rhizomes of C. brunnescens combined with SCT on plant growth, soil fertility, and windbreak and sand dune fixation effects. It is our hope that this study can provide scientific guidelines to explore sand-fixing technology that combines herbs with SCT to recover the degraded vegetation in Maqu alpine region.
The study was conducted in about 9.8 km2area with severe wind erosion and sand burial along the banks of the Yellow River in Maqu County, Gansu Province, northwestern China (33° 06′ 30′ ′ -34° 30′ 15′ ′ N, 100° 45′ 45′ ′ -102° 29′ 00′ ′ E; 3700 m a.s.l.). The study area is characterized by a distinct plateau continental climate with annual mean temperature of 1.1° C, mean annual precipitation of 615.5 mm (atmospheric precipitation), and mean annual potential evaporation of 1353.4 mm (Kang et al., 2015). Dominant plant species include herbs such as C. brunnescens and Kobresia robusta Maxim., and shrubs such as S. oritrepha and Hippophae rhamnoides Linn. (Kang et al., 2016b). The landscape consists of fixed, semi-fixed, and moving sand dunes and C. brunnescensvegetation coverage is > 45%, 15%-30%, and < 15%, respectively (Xu and Liu, 2012; Zhang et al., 2016).
2.2.1 Morphological characteristics of belowground rhizomes of C. brunnescens
In April to September of 2011, we used the randomized complete block design to establish five representative plots (10 m× 10 m each) in the middle parts of the longitudinal semi-fixed and moving sand dunes to determine the morphological characteristics of belowground rhizomes of C. brunnescens. We examined the maximum soil depth of vertical rhizome growth during the vigorous growth period of C. brunnescens in July 2011. Our examination shows that 50 cm is the maximum depth of sand burial for vertical rhizomes of C. brunnescens to grow upwards and to form new ramets. Subsequently, we excavated the belowground rhizomes (to a soil depth of 50 cm) in each plot with three replicates, removed the attached soil by hand shaking, and classified the rhizomes into vertical rhizomes and horizontal rhizomes. We further distinguished two sub-types within the horizontal rhizomes: branching rhizomes and main rhizomes. It should be pointed out that we were not able to completely excavate all horizontal rhizomes in each plot due to the intricacy of the rhizomes. Finally, we measured the length and internode length of the collected vertical rhizomes and horizontal rhizomes (including branching rhizomes and main rhizomes) and counted the number of rhizomes.
2.2.2 Reproductive characteristics of belowground rhizomes of C. brunnescens
Reproductive characteristics of belowground rhizomes of C. brunnescens were observed in a manner similar to that used for the determination of morphological characteristics. Specifically, we used the randomized complete block design to establish four representative plots (5 m× 5 m each) in longitudinal semi-fixed sand dunes to determine the reproductive characteristics of belowground rhizomes of C. brunnescens. We firstly examined the maximum soil depth that belowground rhizomes could survive (0-200 cm). Our examination shows that 120 cm is the maximum depth that belowground rhizomes of C. brunnescens can reach. Then, we divided soil depth of 0-120 cm into 3-4 layers based on the average length (30-40 cm) of vertical rhizomes and classified the rhizomes into vertical rhizomes and horizontal rhizomes (including branching rhizomes and main rhizomes) for each layer. Finally, we observed the reproductive characteristics of belowground rhizomes of C. brunnescens based on the growth performance (including length of rhizomes, internode length of rhizomes, and bud number of rhizome nodes) of vertical and horizontal rhizomes.
2.2.3 Relationships of vegetation parameters with wind erosion and sand burial
When wind erosion was more severe in early April 2011, we observed growth performance (length of rhizomes, internode length of rhizomes, and bud number of rhizome nodes) of C. brunnescens in the direction perpendicular to wind direction. Correspondingly, we determined the spread direction ofC. brunnescens populations for each plot. Then, we used the randomized complete block design to establish ten representatives and replicated belt transects (width of 1 m and length of 17 m) along the spread direction ofC. brunnescenspopulations in longitudinal semi-fixed sand dunes. Among which, five belt transects were used to quantitatively investigate the relationships of population density with wind erosion height and sand burial depth, and the other five were used to establish the relationships of vegetation cover with wind erosion height and sand burial depth. These determinations were conducted once a month from April to September, 2011.
Population density was obtained by excavating all plants in each plot (1 m× 1 m) and counting the ramets. Vegetation cover (projected cover; %) was calculated using the digital imagery (Cailson and Ripley, 1997). Then, sampling points were counted and numbered from photographs and geomorphological characteristics and vegetation status were recorded in April and May. The captured vegetation photographs were adjusted to size (with the removal of length and width of up to 2/3 of the original size) by Photoshop 7.0 software to reduce the errors caused by image edge distortion (Diao et al., 2012).
Wind erosion height and sand burial depth were determined for every wind event in the windy season (April and May) by placing soil sample of known mass in a pan-shaped container (wind erosion pan) and later measured by reweighing the samples (Dai et al., 2011).
We used the randomized complete block design to establish five representative belt transects (width of 1 m and length of 15 m) along the spread direction ofC. brunnescenspopulations in longitudinal semi-fixed sand dunes. Then, the relationships of total length of belowground rhizomes, total length of belowground first-order roots, fresh weight of aboveground part and fresh weight of belowground part with wind erosion height and sand burial depth were quantitatively determined for every month from April to September, 2011.
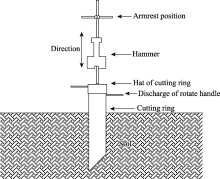
The sampling methods were referred to those described by Kang et al. (2014). Schematic diagram of root sampling instrument is shown in Figure 1. Specifically, along the spread direction ofC. brunnescenspopulations in longitudinal semi-fixed sand dunes, a cutting ring (diameter of 0.15 m and height of 0.5 m) was hammered into the ground vertically allowing for excavation at different soil depths in each quadrat (width of 1 m and length of 1 m) of the belt transect (width of 1 m and length of 17 m), and the ring was then extracted from the soil. The process repeated and transferred to other quadrats. Thus, a large volume of undisturbed soil cores were collected and the characteristics of belowground rhizomes and roots were observed. The aboveground and belowground parts were carefully separated after removing the soil attached to the rhizomes and roots by hand shaking, and their fresh weights were obtained by weighing. Finally, all the belowground rhizomes and roots along the spread direction of C. brunnescens populations were collected to calculate the total length of belowground rhizomes and total length of belowground first-order roots in each quadrat.
2.2.4 Transplanting experiment with the straw checkerboard technique (SCT)
To investigate the effects of SCT on the growth and reproductive performance of belowground rhizomes of C. brunnescens as well as on the soil fertility of C. brunnescens plantations, we conducted a transplanting experiment on sand dunes along the bank of the Yellow River from April 2012 to September 2015. Three experimental sites were designed: sand dunes that had no further treatment (SD+N), sand dunes that had SCT but no transplanted rhizomes of C. brunnescens (SD+SCT), and sand dunes that had both SCT and transplanted rhizomes of C. brunnescens (SD+SCT+P). There were 20 replicated plots in each experimental site. For the straw checkerboard treatments, wheat straw was placed in a 1 m× 1 m checkerboard pattern, and this pattern was reported to be able to achieve remarkable windbreak and sand dune fixation effects (Qiu et al., 2004; Ma et al., 2015). For transplanting of rhizomes of C. brunnescens, healthy and living belowground rhizomes with the same length (25 cm) were collected and placed in a wet plastic bag and then transported to the transplanting sites. We established the transplanting plots in the middle of the longitudinal sand dunes (with application of SCT). In SD+SCT+P experimental site, each transplanting plot (1 m× 1 m; 16 rhizomes per plot covered with 20 cm of wet sand) was surrounded by a buffer zone of 0.5 m. Newly-generated ramets were harvested from each transplanting plot by digging in September 2013, 2014, and 2015. The aboveground and belowground parts were carefully separated and washed three times with water for further morphological analysis.
2.3.1 Growth-related parameters of C. brunnescens
Ramet height was measured with a measuring tape (0.1 m precision). Reproductive ramets and belowground rhizomes were excavated, and the total number was counted in each experimental plot. Total length of belowground rhizomes and total length of belowground first-order roots in each quadrat were calculated by Equation 1. Fresh weight of aboveground part and fresh weight of belowground part in each quadrat were calculated by Equation 2. Survey of plant reproductive characteristics included structural characteristics of rhizomes, source and the maximum sand burial depth from which vertical rhizomes emerged to form new ramets.
$L={{L}_{1}}\times \frac{0.5}{\pi \times {{(d/2)}^{2}}\times h}, $ (1)
$W={{W}_{1}}\times \frac{0.5}{\pi \times {{(d/2)}^{2}}\times h}, $ (2)
where, L (cm) is the total length of belowground rhizomes or total length of belowground first-order roots in each quadrat; L1 (cm) is the total length of belowground rhizomes or total length of belowground first-order roots in each cutting ring; d (m) is the diameter of the cutting ring; h (m) is the height of the cutting ring; W (g) is the fresh weight of aboveground part or fresh weight of belowground part in each quadrat; and W1(g) is the fresh weight of aboveground part or fresh weight of belowground part in each cutting ring, which was obtained by weighing (0.001 g precision).
2.3.2 Soil nutrients
Soil samples were collected in each experimental site (i.e., SD+N, SD+SCT and SD+SCT+P) after all the newly-generated ramets were harvested in September 2015. Specifically, 4-6 subsamples were respectively collected at three soil layers (0-15, 15-30, and 30-50 cm) in each plot, then subsamples from the same soil layer in each plot were mixed into a composite sample. Soil samples were placed in plastic bags and taken to the laboratory for soil chemical analyses. These samples were air-dried at room temperature for 30 d and sieved by a 2-mm mesh. Soil organic carbon (SOC) was measured following the K2Cr2O7-H2SO4 oxidation method of Walkley and Black (Kang et al., 2015), total nitrogen (TN) was measured following the Kjeldahl digestion and total phosphorus (TP) was determined by a UV-1601 visible spectrophotometer (Bao, 2000; Xing et al., 2013). In addition, soil available nitrogen (AN) was determined by the alkaline diffusion method, available phosphorus (AP) was determined by the Bray method, and available potassium (AK) was measured using the ammonium acetate extract method (Bao, 2000).
2.3.3 Soil microbial biomass carbon, nitrogen, and phosphorus
For each experimental site (i.e., SD+N, SD+SCT and SD+SCT+P), five replicated fresh soil samples were collected in each plot. Those soil samples were placed in sealed plastic bags, taken to the laboratory and stored at 4° C. Then, they were fumigated with chloroform after removal of plant residues and other debris. Soil microbial biomass carbon (MBC) was measured by titration with 0.5 mm/L K2SO4 extract, microbial biomass nitrogen (MBN) was determined using the Kjeldahl procedure, and microbial biomass phosphorus (MBP) was measured with the NaHCO3 extraction method after soil fumigation with chloroform (Brookes et al., 1982; Kang et al., 2015).
In this study, we used one-way analysis of variance (ANOVA) for SPSS 13.0 (SPSS Inc., Chicago, IL., USA) to determine the treatments-resulted differences in growth-related parameters of C. brunnescens (such as ramet height, total number of reproductive ramets, fresh weight, etc.) and in soil properties (such as SOC, TN, TP, TK, etc.). Duncan’ s multiple range tests for a completely randomized design were used to detect significant differences in treatment means at P< 0.05 level.
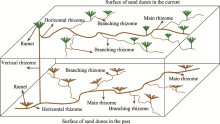
Observations of profile characteristics of C. brunnescen in semi-fixed sand dunes showed that the belowground rhizomes were highly developed and exhibited complex horizontal rhizomes (including branching rhizomes and main rhizomes) and vertical rhizomes (Fig. 2). Ramets were mainly reproduced by the horizontal rhizomes. During the growing season, the ground surface of sand dunes was covered with sands, and the vertical rhizomes that were created by the nodes of the continuously-expanding horizontal rhizomes emerged through the sand surface to form new ramets. The process repeated and then formed a multi-tiered network that stabilized (fixed) the sand dunes. The network of belowground rhizomes formed 4 tiers in a 120-cm sand depth. The maximum sand burial depth from which vertical rhizomes emerged to form new ramets was less than 50 cm.
In semi-fixed sand dunes, the average internode length was 3.0 cm with 10 buds on vertical rhizomes (sand burial depth of 50 cm); while in moving sand dunes, the average internode length was 2.5 cm with 10 buds on vertical rhizomes (sand burial depth of 50 cm) (Table 1). For branching rhizomes, the average internode lengths were 2.5 and 2.6 cm in semi-fixed and moving sand dunes, respectively; while for main rhizomes, the average internode lengths were 4.0 and 4.1 cm in semi-fixed and moving sand dunes, respectively. There was a significant difference in the average internode length between branching rhizomes and main rhizomes in both semi-fixed and moving sand dunes (Table 1).
| Table 1 Morphological characteristics of belowground rhizomes of C. brunnescens in semi-fixed and moving sand dunes |
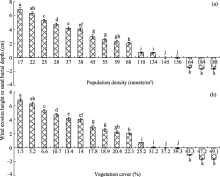
Population density and vegetation cover of C. brunnescens greatly influenced wind erosion height and sand burial depth (Fig. 3). Generally speaking, wind erosion heights decreased with increases in population density (0-145 ramets/m2) and vegetation cover (1.5%-29.2%). It should be mentioned that wind erosion was absent at population density of 145-156 ramets/m2and vegetation cover of 31.2%-39.3%. Furthermore, apparent sand burial was observed at population density > 156 ramets/m2and vegetation cover > 39.3%.
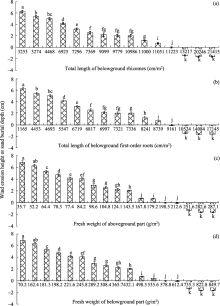
For C. brunnescens, the total length of belowground rhizomes, total length of belowground first-order roots, fresh weight of aboveground part and fresh weight of belowground part exerted great influences on wind erosion height and sand burial depth (Fig. 4). Wind erosion heights decreased with increases in total length of belowground rhizomes (0-11, 051 cm/m2), total length of belowground first-order roots (0-8739 cm/m2), fresh weight of aboveground part (35.7-179.2 g/m2) and fresh weight of belowground part (70.2-535.6 g/m2). It should be mentioned that wind erosion was not observed under following conditions: total length of belowground rhizomes being 11, 223 cm/m2, total length of belowground first-order roots being 9161 cm/m2, fresh weight of aboveground part being 198.5-212.6 g/m2and fresh weight of belowground part being 578.8-612.4 g/m2. Furthermore, apparent sand burial occurred under following conditions: total length of belowground rhizomes > 11, 223 cm/m2, total length of belowground first-order roots > 9161 cm/m2, fresh weight of aboveground part > 212.6 g/m2 and fresh weight of belowground part > 612.4 g/m2.
As shown in Table 2, applying SCT in sand dunes and transplanting belowground rhizomes of C. brunnescens to sand dunes with SCT significantly improved the growth performance of C. brunnescens. In September 2013, the values of ramet height, total number of reproductive ramets, total number of belowground rhizomes, total length of belowground rhizomes, and fresh weight of C. brunnescens were 39.6%, 323.5%, 64.7%, 69.3%, and 123.1% higher in SD+SCT+P than in SD+N, respectively. And, those values were 21.8%, 141.1%, 28.3%, 18.0%, and 42.8% higher in SD+SCT+P than in SD+SCT, respectively. The effects of applying SCT in sand dunes and transplanting belowground rhizomes to sand dunes with SCT on the growth and reproductive performance of C. brunnescens were also confirmed in September 2014 and 2015.
| Table 2 Growth-related parameters of C. brunnescens in three experimental sites in different periods |
Applying SCT in sand dunes and transplanting belowground rhizomes of C. brunnescens to sand dunes with SCT had significant effects on soil nutrient properties (Table 3). Soil nutrient contents (0-15 cm soil depth) significantly increased in sand dunes after the application of SCT and establishment of belowground rhizomes of C. brunnescens. Specifically, the contents of SOC, AN, AP, and AK were 50.4%, 78.4%, 63.1%, and 131.9% higher in SD+SCT than in SD+N, respectively. And, those values were 34.5%, 23.5%, 43.0%, and 25.4% higher in SD+SCT+P than in SD+SCT, respectively. Similar variation trends were also observed in 15-30 and 30-50 cm soil depths. Furthermore, SOC, AN, AP, and AK contents exhibited generally decreasing trends with increasing soil depth from April 2012 to September 2015.
| Table 3 Soil nutrient properties at different soil depths in three experimental sites from April 2012 to September 2015 |
Transplanting belowground rhizomes of C. brunnescens to SD+SCT experimental site significantly affected soil MBC, MBN, and MBP. As shown in Figure 5, from April 2012 to September 2015, the contents of MBC, MBN, and MBP (0-15 cm depth) in SD+SCT+P increased by 33.1%, 48.2%, and 38.3% compared to those in SD+SCT, and by 75.2%, 102.9%, and 125.4% compared to those in SD+N, respectively. Similar variation trends were also found in 15-30 and 30-50 cm soil depths during this period. Furthermore, MBC, MBN, and MBP contents generally decreased with increasing soil depth at each experimental site.
Clonal plants are special types of plants that can maintain normal survival and reproduction in sand dune environments. During the evolutionary process, clonal plants have acquired many adaptive mechanisms to tolerate sand burial and wind erosion stresses (Liu et al., 2007; Yu et al., 2008; Tang et al., 2010; Ye and Dong, 2011). In this study, we determined that belowground rhizomes of C. brunnescens developed structures with a strong ability to grow upward and to form new ramets when sand burial depth was < 50 cm (see Table 1), indicating that belowground structures of C. brunnescens may be an inevitable evolution under wind stresses (Kang et al., 2016a, b; Ma et al., 2017). Moreover, our results showed that C. brunnescens growing in sand dunes with application of straw checkerboard technique (i.e., SD+SCT) can produce more clonal ramets than that growing in sand dunes that had no further treatment (SD+N), and this trend can be further increased after the establishment of belowground rhizomes of C. brunnescens. This may be due to the positive effects of wind erosion and sand burial on ramet growth and regeneration of C. brunnescens (see Table 2). Interestingly, our previous studies have shown that C. brunnescens generated more ramets in simulated blowouts than in natural conditions and that the sources of new ramets were dormant buds and buds spreading from outside (Kang et al., 2016a). Dormant buds were released from dormancy in the deeper soil layers under wind erosion conditions, suggesting that C. brunnescens has a strong potential for blowout remediation (Kang et al., 2016a). It seems that C. brunnescensshows excellent capacity for sand fixation through rapid clonal expansion that ensures its rapid growth and reproduction. This finding provides a new perspective for improving clonal plants for biomass production and the restoration of degraded vegetation in Maqu alpine region. However, it is still unknown why C. brunnescens grows well in these stressful conditions without any detectable signs of stress from wind erosion or sand burial. The answers may involve division of labor and ramet specialization as important parts of clonal integration in C. brunnescens (Xu et al., 2010; Luo et al., 2015; Luo and Zhao, 2015). Spatial division of labor among ramets that are connected with each other has a strong ability to share resources (including water, carbon, nitrogen, and phosphorus), and ramet specialization and cooperation in resource uptake are beneficial to plant biomass and clonal regeneration of C. brunnescens at small spatial scales (Roiloa et al., 2007, 2014; Luo and Zhao, 2015; Kang et al., 2016a). Therefore, our results further confirmed that clonal integration and reproduction may be extremely important to the survival and regeneration of C. brunnescens in building resistance and resilience to the unstable sand dune environments (Xu et al., 2010; Luo et al., 2015; Kang et al., 2016a). However, the physiological and ecological adaptive mechanisms still need a further study.
Wind erosion and sand burial are the most common abiotic stresses for plant growth in the desert areas of northwestern China. Therefore, the choice of well-adapted plant species for the restoration and stabilization of moving sand dunes is of critical importance (He et al., 2007; Gong et al., 2014; Wang et al., 2016). When vegetation cover decreases, the protective effect of vegetation on surface soil decreases, the exposed part of the surface soil increases, and the small particles of surface soil can be readily blown into the air under the action of strong winds (Gu et al., 2002; Zhang et al., 2016). A significant negative correlation between vegetation cover and wind erosion has been previously documented (Hai et al., 2002; Xu and Liu, 2012; Zhang et al., 2016). Our results showed that forC. brunnescens species, following conditions were well-documented to have great effects on wind erosion and sand burial (see Figs. 3 and 4): population density at 145-156 ramets/m2, vegetation cover at 31.2%-39.3%, total length of belowground rhizomes at 11, 223 cm/m2, total length of belowground first-order roots at 9161-10, 524 cm/m2, fresh weight of aboveground part at 198.5-212.6 g/m2, and fresh weight of belowground part at 578.8-612.4 g/m2. Understanding the vegetation characteristics (composition, structure, and function) of C. brunnescens and addressing the relationships of growth-related characteristics (population density, plant cover, and aboveground and belowground parts) of C. brunnescens with wind erosion and sand burial have important theoretical significance for the prevention and control of soil wind erosion. More importantly, the population and community structure, and configuration and succession of belowground structures of C. brunnescens need to be explored in-depth for promoting natural succession and also for restoring degraded vegetation in Maqu alpine region. To sum up, C. brunnescens can be selected for ecological restoration and soil erosion control in Maqu alpine region.
It is well known that soil erosion is an important process that affects both surface features and biological potential of soils (Wezel et al., 2000; Ye and Dong, 2011; Xu et al., 2012). Sand fixation with plants is considered to be the most effective measure in controlling desertification (Wang et al., 2016). However, vegetation in sand dunes can be easily disturbed by environment changes and human activities. As self-recover ability of vegetation in sand dunes is very weak, a combination of biological and engineering measures is recommended to improve the effect of vegetation on sand stabilization (Dai et al., 2008; Zhang et al., 2014). Previous studies have widely reported that establishment of stable artificial sand-fixing plant communities with SCT is an effective strategy for sand fixation and for soil and water conservation in many arid and semi-arid regions of China (Qiu et al., 2004; Ma et al., 2015). For moving sand dunes, the application of SCT with plants not only stabilizes the surface of sand dunes but also improves soil nutrient properties through belowground root systems and inputs of organic matter from decomposing straw, dead branches, and fallen leaves of plants. It was previously reported that some shrub plants (e.g., Caragana microphyla, Hedysarum fruticosumand Salix oritrepha) and herbs (e.g., Agropyron cristatum) could be planted with SCT to further improve the windbreak and sand dune fixation effects (Qiu et al., 2004; Cao et al., 2007; Zhao et al., 2010; Zhu et al., 2012; Kang et al., 2015). In our study, transplanting belowground rhizomes ofC. brunnescens to SD+SCT not only significantly promoted the growth and reproductive performance of C. brunnescens (Tables 2 and 3) but also increased soil nutrient contents. Our results indicated that the establishment of straw checkerboard in sand dunes increases soil MBC, MBN, and MBP contents, and these contents can be further increased by establishing belowground rhizomes ofC. brunnescens (see Fig. 5). This may be associated with the organic and inorganic substances of plant matter in the soil that provide nutrition and energy for a variety of microorganisms and are conducive to the growth and reproduction of microorganisms.
Understanding the vegetation characteristics (composition, structure, and function) of C. brunnescens and addressing the relationships of growth-related characteristics (population density, plant cover, and aboveground and belowground parts) of C. brunnescens with wind erosion and sand burial have important theoretical significance for the prevention and control of soil wind erosion. The efficient vegetative reproduction and high productivity of C. brunnescens in alpine sandy environment are resulted from the highly-developed and complex belowground horizontal rhizomes that can continuously create vertical rhizomes emerging from sand surface and forming new ramets. Our observations demonstrated that transplanting belowground rhizomes of C. brunnescens to SCT-controlled sand dunes has pronounced windbreak and sand fixation effects in degraded alpine sandy environments. Our results further confirmed that clonal integration and reproduction may be extremely important to the survival and regeneration of C. brunnescens in building resistance and resilience to the unstable sand dune environments. However, the physiological and ecological adaptive mechanisms still need a further study. It is also worth mentioning that due to the high seed dormancy of C. brunnescens in natural conditions (seed germination rate < 14%), the practice of using seed reproduction may limit the ability to cultivate C. brunnescens under SCT-controlled sand dunes in the degraded alpine meadow of Maqu. Therefore, the sand fixing principle, methods for breaking seed dormancy, seed germination, and dormancy mechanisms of C. brunnescens also require a further research.
This work was supported by the National Natural Science Foundation of China (31360087, 31360086). The authors are very grateful to Dr. Kathryn PIATEK (a professional translator and editor in USA), as well as the anonymous reviewers and editors for their critical review and comments which helped to improve and clarify the paper.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|