The first and second authors contributed equally to this work.
Monitoring soil microbial communities can lead to better understanding of the transformation processes of organic carbon in soil. The present study investigated the changes of soil microbial communities during straw decomposition in three fields, i.e., cropland, peach orchard and vineyard. Straw decomposition was monitored for 360 d using a mesh-bag method. Soil microbial metabolic activity and functional diversity were measured using the Biolog-Eco system. In all three fields, dried straws with a smaller size decomposed faster than their fresh counterparts that had a larger size. Dried corn straw decomposed slower than dried soybean straw in the early and middle stages, while the reverse trend was found in the late stage. The cropland showed the highest increase in microbial metabolic activity during the straw decomposition, whereas the peach orchard showed the lowest. There was no significant change in the species dominance or evenness of soil microbial communities during the straw decomposition. However, the species richness fluctuated significantly, with the peach orchard showing the highest richness and the cropland the lowest. With different carbon sources, the peach orchard utilised carbon the most, followed by the cropland and the vineyard. In all three fields, carbon was utilized in following decreasing order: saccharides>amino acids>polymers>polyamines>carboxylic acids>aromatic compounds. In terms of carbon-source utilization, soil microbial communities in the peach orchard were less stable than those in the cropland. The metabolic activity and species dominance of soil microbial communities were negatively correlated with the straw residual percentage. Refractory components were primarily accumulated in the late stages, thus slowing down the straw decomposition. The results showed that dried and crushed corn straw was better for application in long-term fields. The diversity of soil microbial communities was more stable in cropland than in orchards during the straw decomposition.
Straws contain nitrogen, phosphorus, and potassium, and many other elements necessary for plant growth (Arcand et al., 2014). Proper straw application to soils is of eco-environmental importance to agricultural production (Han et al., 2008; Ibrahim et al., 2015). Specifically, straw application can maintain soil quality by reducing fertiliser use, improving the soil carbon sink capacity, promoting the soil nitrogen cycle and preventing combustion-resulted environmental pollution (Gale and Cambardella, 2000; Pan et al., 2013; Arcand et al., 2014). However, straw application may eventually generate negative effects during straw decomposition stage and the negative effects include the changes of the soil carbon to nitrogen (C/N) ratio, accumulation of organic acids, reduction of mineral nitrogen (Martens, 2000; Yan et al., 2012; Ma et al., 2013; Yang et al., 2014). Therefore, scientific efforts are needed to make straw decomposition in soil harmless.
The rate of straw decomposition in the soil is influenced by the size and type of crop residues (Toenshoff et al., 2012; Wang et al., 2012; Wang and Sun, 2012; Iqbal et al., 2014), as well as by temperature, moisture, and soil properties (Wang et al., 2009; Toenshoff et al., 2014). Soil microorganisms, as the most active components in soil ecosystems, are sensitive indicators in reflecting the changes in the soil micro-environment (Lundquist et al., 1999). Soil microbial communities influence soil nutrient cycling and energy flow, and nutrient cycling and energy flow are able to affect soil ecosystem functions (Dong et al., 2012). The functional diversity of soil microbial communities can be determined using a Biolog-Eco microplate, which utilises a sole carbon-source to reflect the different metabolic functions of microorganisms (Li et al., 2011). Biolog-Eco microplates are useful to directly monitor the carbon-source utilization pattern of soil microorganisms and preliminarily determine the changes in straw-degrading microorganisms during straw decomposition (Wang et al., 2012; Wang and Sun, 2012).
Recent attention of plant residue decomposition studies have been devoted to the effects of incorporating pre-treated straws into fields on soil nutrients and labile organic carbon (Ma et al., 2013), also to the impacts of climate change and fertilisation on the metabolic characteristics of soil microbial communities (Kong et al., 2009; Dong et al., 2012), and additionally to the influence of amended inorganic nitrogen and glucose on soil microbial biomass and activity (Yu and Zhao, 2012). However, less attention has been paid to the changes of soil microbial communities during the decomposition of various crop straws (e.g., corn and soybean) in long-term farming fields in arid and semi-arid areas.
In the present study, we investigated the changes in metabolic activity and functional diversity of soil microbial communities during corn and soybean straw decomposition in three long-term fields, i.e., cropland, peach orchard and vineyard. We also monitored the changes in straw residual content, soil temperature and water content, in order to elucidate their interactive relationships with soil microbial activity and diversity.
The experiment was carried out on three long-term fields, i.e., a cropland, a peach orchard and a vineyard with 23, 12 and 11 years of operating history, respectively. The cropland field is located at the National Soil Fertility and Fertiliser Effects Long-term Monitoring Site (34° 17′ 51″N, 108° 00′ 48″E) in the southern part of the Loess Plateau in Wuquan Town, Yangling District, Shaanxi Province, China. The elevation is 524.7 m, the annual mean temperature is 13° C and ≥ 10° C of accumulated temperature is 4196° C. The mean annual precipitation is 550-600 mm and mainly concentrated from July to September. The average annual evaporation is 993 mm with the frost-free period being 184-216 d. Both the peach orchard and vineyard fields are located in an experimental site (34° 29′ 81″N, 108° 07′ 11″E) of Northwest A& F University (Yangling, China). The climatic conditions of the peach orchard and vineyard are the same as the long-term monitoring site.
The cropland was planted with winter wheat-summer corn rotation, whereas both the peach orchard and vineyard were planted with mulching. All the soils were Earth-cumuli-Orthic Anthrosols (Loutu soil) according to the Chinese Soil Taxonomy (Chang et al., 2001). The basic soil properties in the three experimental fields are shown in Table 1. The soil pH in all three experimental fields was 8.2. The cropland soil had a significantly greater amount of nitrogen than the other two fields, while the readily available potassium in the peach orchard soil was nearly two times higher than that in the cropland soil. The vineyard soil, however, had the highest available phosphorous.
| Table 1 Properties of the soil in the three experimental fields |
Corn straw was collected from the experimental cropland soil and soybean straw was collected from the adjacent cropland soil. The basic chemical properties of the untreated straws were analysed using conventional methods (Bao, 2000) and the results are shown in Table 2. The total carbon in the corn straw was greater than that in the soybean straw, while the total nitrogen was greater in the latter (i.e., soybean), resulting in a significantly higher C/N ratio in corn straw. The fresh straws that contained a significant amount of water were crushed to 1-2 cm in size and immediately stored in the refrigerator at 4° C. The other straws were first air-dried and then oven-dried at 60° C to a constant weight, and eventually crushed to < 1 mm before use.
| Table 2 Chemical properties of corn and soybean straws before decomposition |
A total of six straws were investigated, i.e., fresh corn straw (FC), fresh corn straw+nitrogen (FCN), dried corn straw (DC), dried corn straw+nitrogen (DCN), fresh soybean straw (FS) and dried soybean straw (DS). Straws were weighed and packed into 18 cm× 12 cm double 350 mesh nylon net bags. The fresh and dried straws were packed at 30 and 15 g per bag, respectively. The bags were sealed and buried into the soil at a depth of 20 cm and a spacing of 20 cm. There were 90 bags per treatment and 540 bags per field. Soil samples were manually collected within 1 cm of the nylon net bag for physical-chemical and microbial analysis. Triplicate samples were collected at 10, 20, 30, 45, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330 and 360 d. A soil temperature logger (TidbiT v2, USA) was buried at the same depth as the nylon net bags (i.e., 20 cm). Soil temperature was recorded every hour.
The straw bags were weighed before being buried into the soil and late retrieved when soils were sampled. Fresh samples were weighed directly after retrieval from the fields while dried samples were weighed after drying at 60° C to a constant weight. The residual percentage of straw decomposition was calculated according to the following equation: straw residual percentage (%)=Xt/X0× 100%, where X0 is the initial mass of straw before decomposition and Xt is the residual mass after decomposition at time t. The change in straw residual percentage over time was used to measure the rate of straw decomposition.
The catabolic diversities of the soil microbial communities were estimated using Biolog-Eco microplates (Biolog Co., Hayward, CA, USA). The Biolog-Eco system was comprised of 31 carbon sources (classified as six categories) and also of one control well without any carbon-source. Three replicates of fresh soil samples (5 g dry weight) were dissolved in 45 mL sterile 0.85% NaCl and shaken at 180 r/min for 30 min. The suspension was stepwise diluted 1000 times using sterile 0.85% NaCl solution. Subsequently, 150 µ L diluted suspensions were inoculated into the Eco-microplate and incubated at 28° C for 240 h. The carbon-source utilization was daily measured using a Biolog MicrostationTM (BIO-TEK Instruments Inc., Winooski, VT, USA) at 590 nm according to the manufacturer’ s instructions (Qian et al., 2014).
The metabolic activity of the soil microbial community was calculated according to the average well colour development (AWCD): AWCD=∑ (Ci-R)/31, where Ci is the absorbance of the carbon-source and R is the absorbance of the control well. AWCD indicates the total metabolic capacity of soil microbial community in terms of carbon-source utilization (Zabinski and Gannon, 1997). A higher AWCD value indicates a higher microbial metabolic activity and an utilization density of sole carbon source by microorganisms. As 144 h is the inflection point of the AWCD curve (Jia et al., 2013), we collected the results at 144 h to calculate the Shannon’ s diversity index (H), Simpson’ s diversity index (D) and Shannon’ s equitability (E), which characterise the species richness, dominance and evenness in a community, respectively (Wang et al., 2012).
A two-way ANOVA was conducted using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Data are means± standard deviations (n=3).
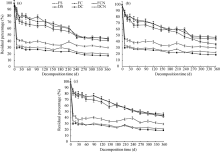
3.1.1 Straw residual percentages
The straw residual percentages decreased as a function of time and showed similar trends in all three fields (Fig. 1). The residual percentage of the dried straws (36.1%-90.3%) was significantly greater than that of the fresh straws (17.6%-44.5%) over the entire study period. Judged from the changes in straw residual percentages, FS, FC and FCN were decomposed rather fast within the first 10 d. The residual percentages of DS, DC and DCN kept dropping for 240 d and then maintained at a relatively stable level.
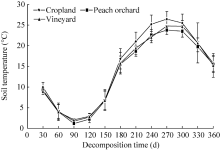
3.1.2 Soil temperature
The mean soil temperature in the cropland (1.72° C-26.45° C) was slightly higher than those in the vineyard (1.16° C-23.79° C) and peach orchard (2.09° C-24.78° C) (Fig. 2). The lowest and highest temperatures were observed at 90 and 270 d, respectively. Soil temperature deviations in the peach orchard and vineyard were smaller than that in the cropland (Fig. 2).
 | Fig. 2 Variations in mean soil temperature in the three experimental fields. Bars indicate standard deviations. |
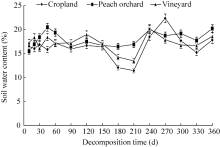
3.1.3 Soil water content
The peach orchard with lush foliage had a significantly higher (P< 0.01) soil water content (18.01%) compared with the vineyard (17.21%) and cropland (16.41%) (Fig. 3). The soil water contents of the cropland and vineyard fluctuated slightly during the initial decomposition period (0-90 d), while the peach orchard experienced the most significant decrease from 60 to 90 d. During 120-210 d, the most significant reduction in soil water content was observed in the cropland, followed by the vineyard. The soil water content of the peach orchard, however, did not change significantly. After 210 d, the soil water content of the cropland increased dramatically from 11% (210 d) to 23% (270 d) and subsequently dropped. Although an increase was also observed in both the cropland and vineyard from 210 to 240 d, this was followed by a general decrease. From 330 to 360 d, all three fields experienced similar increases.
3.2.1 Average well colour development (AWCD)
The AWCD values of the cropland and peach orchard increased in the initial stage of 0-120 d (Fig. 4) with a slight drop at 90 d. The AWCD values of the vineyard, however, increased from 0 to 30 d and then declined gradually until 90 d. The decline in soil microbial activity between 30 and 90 d seemed to be associated with the low soil temperature during this period. After 120 d, the AWCD values of all three soils fluctuated to varying degrees. The final AWCD values of the cropland, vineyard and peach orchard soils were observed to increase by 103.78%, 29.52% and 9.28%, respectively, compared with the initial values. The AWCD values of the three soils at 144 h were not significantly different (P> 0.05), while the differences after 144 d were highly significant (P< 0.01). Overall, the average soil microbial activity followed the decreasing order of cropland> vineyard> peach orchard.
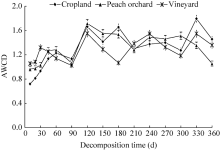 | Fig. 4 Variations of average well colour development (AWCD, 144 h) in the three experimental fields. Bars indicate standard deviations. |
3.2.2 Soil microbial diversity indices
Table 3 shows that the D (species dominance) values of the three fields remained in a narrow range of 0.94-0.96. Furthermore, the D values of the three soils did not change significantly over the decomposition time (P> 0.05). The H (species richness) and E (species evenness) values changed significantly over 0-120 d, but were relatively stable in the remaining period. The average H values were similar in the cropland (3.52± 0.13), peach orchard (3.59± 0.09) and vineyard (3.58± 0.13). The Evalues showed greater fluctuations over the entire decomposition period. On average, the Evalues in cropland (0.98-1.23) were higher than those in the vineyard (1.00-1.18) and peach orchard (1.02-1.14).
| Table 3 Species richness (H), dominance (D) and evenness (E) indices of soil microbial communities in the three experimental fields |
According to the ANOVA results, the differences in H values among the three soils were highly significant (P< 0.01). Overall, the H values followed the decreasing order of vineyard> cropland> peach orchard, and the difference was highly significant between the vineyard and the peach orchard (P< 0.01). However, the Evalues were not significantly different between fields over the decomposition time (P> 0.05).
3.2.3 Carbon-source utilization of soil microbial communities
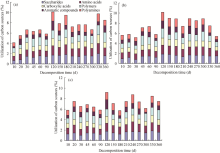
The AWCD value and microbial diversity indices only reflect the overall changes in soil microorganisms. Microbial utilization of different carbon sources may indicate microbial metabolic functional groups. Thus, we analysed carbon-source utilization results based on the 144-h absorbance values, which belong to six categories of carbon sources: amino acids, aromatic compounds, carboxylic acids, polymers, polyamines and saccharides.
The utilization of the six categories of carbon sources in the three fields varied during different decomposition periods (Fig. 5). At the initial stage (0-90 d), the utilization of different carbon sources increased steadily in cropland and changed little in orchards. The soil utilization of carbon sources in the three fields reached the maximum at 120 d. From 120 to 360 d, the utilization of carbon sources first decreased and then increased, followed by a final increase to the original level in the cropland and the vineyard. However, the final increase in the cropland and the vineyard was not observed in the peach orchard.
In comparison with the initial values, the final utilization of the six categories of carbon sources varied as much as 205.47% in the cropland and as little as 95.67% in the peach orchard with an intermediate variation (129.29%) in the vineyard. In the cropland soil, the utilization rates of sugars, amino acids, carboxylic acids, polymers, aromatic compounds and polyamines increased to 235.61%, 182.47%, 155.56%, 216.72%, 448.37% and 144.17%, respectively. In the vineyard, the utilization rates of sugars, amino acids, carboxylic acids, polymers and aromatic compounds increased to 126.40%, 120.52%, 115.27%, 194.88% and 140.87%, respectively, while the utilization of polyamines decreased to 86.28% of the initial value. In the peach orchard, the utilization rate of sugars and polymers increased to 160.07% and 109.63%, respectively, while the utilization rates of amino acids, carboxylic acids, aromatic compounds and polyamines dropped to 85.72%, 84.47%, 67.83% and 62.69% of the initial values, respectively.
According to the statistical analysis, the overall utilization of the six carbons in the three soils followed the decreasing order of peach orchard> cropland> vineyard and the difference was significant (P< 0.05) between the peach orchard and the vineyard. Differences between the three soils were significant (P< 0.01). In all three soils, the six carbons were utilized in the following efficiency decreasing order: saccharides> amino acids> polymers> polyamines> carboxylicacids> aromatic compounds.
The decomposition rate of plant residues depends on their inherent properties and the ambient conditions (Zhang et al., 2014). The size of the plant residue not only affects soil microbial activity, but also influences the exchange of water, oxygen and nutrients between soils and straw residues (Ibrahim et al., 2015). In the present study, dried straws with a smaller size (< 1 mm) decomposed faster than fresh straws with a larger size (1-2 cm) during 10-360 d (Fig. 1). High water content of fresh straws did not necessarily accelerate the decay rates. This may be due to the greater energy requirement for fresh straw decomposition (Zhang et al., 2009). As fresh straws had a larger volume but a smaller surface area, the microbial activities in the fresh straws were relatively lower than those in the dried counterparts (Fig. 1), resulting in a slower decomposition rate for fresh straws (Hassink, 1997). The previous study showed that dried straws had smaller size but larger surface area, so microorganisms and enzymes were more likely to contact with the dried straws and accelerate their decomposition. Thus, different sizes of plant residue can result in various decomposition rates and microbial populations in the soil. The residual percentages of fresh straws were substantially lower than those of dried straws in the first 10 d, probably due to significant straw water loss in the initial stage.
The C/N ratio of plant residues also affects their decomposition rates (Wang et al., 2012). In our study, the FS and FCN samples with a much lower C/N ratio decomposed slower than the FC samples (Fig. 1). The slow decomposition of FS and FCN samples may be attributable to their large sample volume, high water content and low surface area. In the dried straws, the DC samples with a higher C/N ratio had relatively high residual percentage in the early and middle stages (Fig. 1); when a peak of soil microbial activity appeared in the late stage. Our observation is in agreement with the results of Iqbal et al. (2014). Our results showed that straws with a lower C/N ratio and a smaller size decomposed faster in the early stage, while straws with a higher C/N ratio and a smaller size showed an accelerated decomposition rate in the late stage, being supportive to the finding of Wang et al. (2012).
Ambient temperature directly affects the strength, growth and reproduction rate of soil microorganisms, and thus indirectly influences the decomposition rate of plant residues and soil organic matter (Dutzler, 1981; Wang et al., 2004). In the present study, the cropland soil had slightly higher temperature deviations as compared with the peach orchard and vineyard soils (Fig. 2). Higher temperature deviations can easily lead to the instability of soil microbial activity (Bauer et al., 2008). Therefore, the differences in the residual percentage of different straws in the cropland soil were relatively obvious during the entire decomposition period. The temperature deviations of the orchard soil were relatively small, resulting in minor changes in the residual percentages of different straws. Moreover, the mulching system applied to the peach orchard and vineyard may have resulted in greater soil microbial activities and variations between different decomposition stages compared with the winter wheat-summer corn rotation system adopted in the cropland (Marcel van der Marcel and Wagg, 2013).
We found that soil temperatures were negatively correlated with the residual percentages observed for FC, FCN, DS, DC and DCN straws in all three fields. As for FS, the negative correlation was observed only in the peach orchard (Table 4). The cropland and vineyard had greater deviations of soil temperature and lower soil water contents and C/N ratios, therefore probably resulting in unstable soil microbial activities and slow straw decomposition. The peach orchard had relatively smaller deviations of soil temperature and complex root systems, therefore probably resulting in stable soil microbial activities favourable for straw decomposition.
| Table 4 Relationships between straw residual percentages and soil parameters |
Water content is another factor affecting soil microbial growth. Within a certain range of temperature, soil microbial activity and respiration rate increase with soil water content (Li et al., 2011). The most significant changes in soil water content were observed in the cropland, followed by the vineyard and peach orchard (Fig. 3). Due to large and complex root systems in the peach orchard, the soil water content of the peach orchard was relatively higher than those of the cropland and vineyard in the rainy season. Furthermore, the peach orchard soil had grass coverage, so the evaporation of soil moisture was usually less than that in the cropland soil. Therefore, the soil water content in the peach orchard with mulching was on average greater than that in the cropland with winter wheat-summer corn rotation. Soil water content had no correlation with the residual percentage in the three fields, indicating that the soil water content had a minor effect on straw decomposition (Table 4), being consistent with that observed by Zhou et al. (2015).
Microorganisms are the central players in determining soil carbon and nitrogen transformations (Hu et al., 2012). In the present study, the microbial species richness in the cropland soil was lower than that in the orchard soil (Table 3), most likely due to the lower evenness of soil microbial communities under grass coverage in the orchard soil (Sun et al., 2015). The soil microbial communities were more considerably influenced by straw decomposition in cropland soils than in vineyard and peach orchard soils. This was probably due to the slow growth and less nutrient absorption by winter wheat in cropland soil. The robust roots of peach could absorb nutrients continually from soil, thus resulting in less change in soil microbial community during straw decomposition. The decreased species richness and the increased species dominance in all three fields (Table 3) may be attributed to the emergence of straw decomposition-related species (Du et al., 2015).
ANOVA analysis revealed that the microbial species richness changed significantly with decomposition time, whereas the species dominance and evenness had minor changes in the three soils. The greatest utilization of six categories of soil carbons observed at 120 d was probably due to moderate soil temperature and soil water content (Nan, 2010). Correlation analysis indicated that the straw residual percentage was negatively correlated with soil microbial activity (AWCD value), with the microbial species dominance, and also with the utilization of saccharides, amino acids and polyamines (Table 5). Except for the residual percentage of dried straw that was correlated with the microbial species evenness, the straw residual percentage showed correlation neither with the microbial species richness, nor with microbial species evenness, or also nor with the utilization of aromatic compounds. Moreover, the residual percentages of FS, DS, DC and DCN straws were negatively correlated with the utilization of carboxylic acids; while the residual percentages of FS, FC and DS were negatively correlated with the utilization of polymers. According to the total utilization of carbon sources in different decomposition periods (Fig. 5), saccharides, amino acids and polymers were the main carbon sources used in the treatment of soil microbiological utilization (Du et al., 2015). Carboxylic acids and polymers were utilized only during a limited interval of the straw decomposition period. Aromatic compounds were least utilized in the process of straw decomposition. The study showed that the readily decomposed substances gradually decreased with time, being consistent with Zhang et al. (2009).
| Table 5 Relationship between straw residual percentage and soil microbial diversity |
In this study, the Biolog-Eco system was used to monitor the changes of soil microbial communities during straw decomposition in three long-term fields of cropland and orchards. The traditional Biolog-Eco analysis provides a relatively simple, rapid option for studying the structure and functional diversity of soil microbial communities (Lu et al., 2013; Wang et al., 2016). The results can reflect the transformation of soil carbon sources and the metabolic activity of soil microbial communities. However, this approach cannot directly acquire detailed information on the structure of soil microbial communities (Wu et al., 2013). Genetic fingerprinting techniques such as T-RFLP and PCR-DGGE are needed to further explore the differential utilization of carbon sources by soil microorganisms during straw decomposition, and screening preferable microbial populations for straw decomposition.
This study investigated the decomposition of fresh and dried straws and the dynamic changes of soil microbial communities in three long-term fields of cropland and orchards. Dried straws decomposed faster than their fresh counterparts, indicating that straws with a higher water content and larger size are harder to decompose. The soil microbial community was less stable in the peach orchard and vineyard where soil temperature was smaller than in the cropland. Straw residual percentage was negatively correlated with soil temperature, microbial metabolic activity and species dominance, as well as with the utilization of saccharides, amino acids and polyamines. Refractory components accumulated in the late stage, slowing down the rate of straw decomposition. The utilization of six categories of carbon sources during straw decomposition could be used to screen the dominant straw-degrading microbes and improve the decomposition efficiency of refractory straws. This study provides evidence for reasonable use of biomass resources under different land use types in arid and semi-arid areas.
This research was supported by the Soil Erosion and Dryland Farming on Loess Plateau of the State Key Laboratory of Chinese Academy of Sciences (K318009902-1310) and the Shaanxi Province Innovative Engineering Project Coordinator (2011K01-48). We thank Dr. GU Jie, SUN Wei, QIAN Xun, and ZHANG Youwang, College of Resources and Environmental Sciences, Northwest A&F University for sample testing and analysis.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|